Novel staphylococcus protein A and preparation method thereof and application
A staphylococcal protein and affinity technology, applied in the biological field, can solve the problems of low purification efficiency and low affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of recombinant staphylococcal protein A
[0020] 1. Construction of recombinant staphylococcal protein A gene monomer
[0021] By chemical synthesis, design and synthesize a sequence monomer of a repeat fragment of the staphylococcal protein A gene (the sequence of the repeat fragment is shown in the 47th-217th nucleotides shown in SEQ ID NO: 2), which includes a length of 171bp The repeated fragments, and the NcoI restriction site connected to the expression vector, 6 His (for affinity chromatography, combined with nickel column, reducing purification steps), EK restriction site (for His and DDDDK is cut off to form the correct Protein A) and Acll restriction site (AACGTT, used to join multiple repeat fragments). In addition, it also includes the stop codon TAA, and a total of 9 bp of BamH I restriction endonuclease linker for cloning. Labeled "Monomer 1".
[0022] In addition, by means of chemical synthesis, a sequence monomer of a repeat frag...
Embodiment 2
[0036] Example 2 Detection of Recombinant Staphylococcal Protein A and Antibody Binding Activity by ELISA
[0037] ELISA method was used to detect the protein A1 and antibody binding activity prepared in the above-mentioned embodiment 1, and the specific process was as follows:
[0038] 1) Coating: Coat each well of a 96-well plate with 150 μl of Protein A 1 sample, 38°C, 1 h;
[0039] 2) Blocking: each well was blocked with 150 μl of 1% BSA at 38° C. for 1 hour;
[0040] 3) Add primary antibody: Add about 150ug human IgG antibody (purchased from Mlbio) to each well, 38°C, 1h;
[0041] 4) Add secondary antibody: Add about 150 μl, 1:1000 horseradish peroxidase-labeled antibody (purchased from Mlbio Company) to each well, 38°C, 1h;
[0042] 5) Color development.
[0043] The same method was used to detect the binding activity of commercially available Protein A (purchased from Guduo Biotech) and antibody.
[0044] ELISA test results: Protein A 1 of the present invention is com...
Embodiment 3
[0045] Example 3 Preparation of Recombinant Staphylococcal Protein A Sepharose Affinity Chromatography Medium
[0046] Utilize the Protein A1 of embodiment 1 to prepare agarose gel affinity chromatography medium, comprise the following steps:
[0047] 1. Activation of agarose gel: react in aqueous medium with epichlorohydrin, sodium hydroxide and agarose (5% cross-linked agarose gel), and react 2- After 3 hours, after the reaction, wash with distilled water until neutral and then drain to obtain activated agarose gel;
[0048] 2. Chemical coupling of recombinant staphylococcal protein A to activated agarose gel: react the activated agarose gel obtained in the above step 1 with the Protein A1 prepared in Example 1 at a temperature of 5-25°C for 15-20 hours After the reaction, it was washed and dried to obtain staphylococcal protein A sepharose affinity chromatography medium.
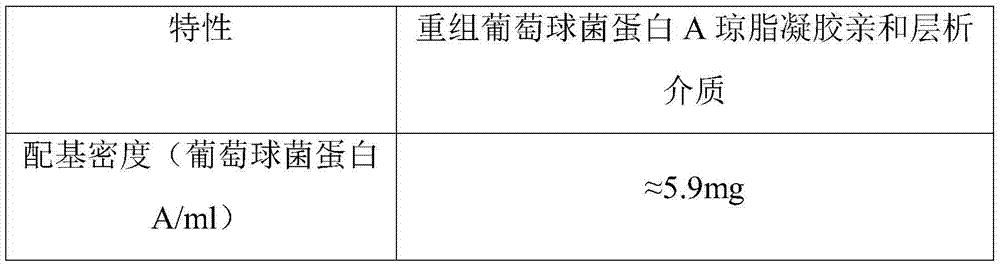
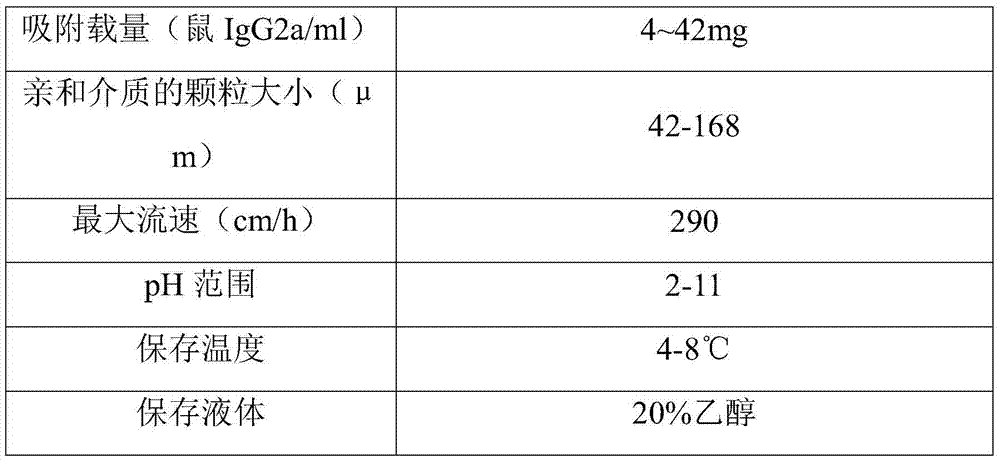
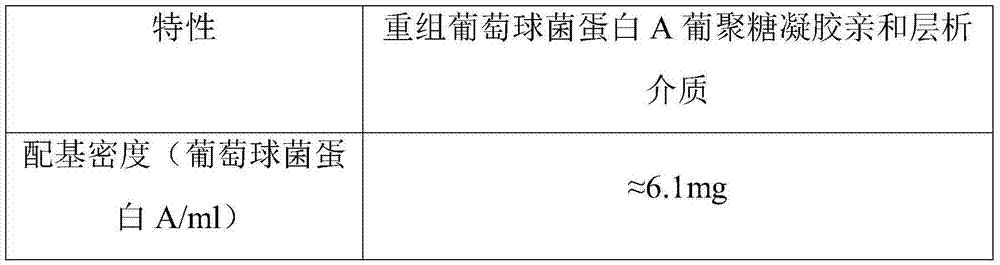
[0049] After testing, the characteristics of the prepared recombinant staphylococcal protein A Sepha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com