Preparation method of recombinant protein A
A technology of recombinant proteins and recombinant strains, applied in the biological field, can solve the problems of low affinity and low purification efficiency, and achieve the effects of high affinity, high expression efficiency and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the synthesis of recombinant protein A gene monomer
[0026] By means of chemical synthesis, the sequence of a repetitive fragment of the recombinant protein A gene was designed and synthesized. The sequence included a length of 168 bp, and the NcoI restriction site connected to the expression vector was added to the front end, and 6 His (for affinity chromatography, and Nickel column combination, reducing purification steps) and EK restriction site, and AccI restriction site for ligation of multiple repeat fragments. In addition, it also includes the stop codon TAA, and a total of 9 bp of BamH I restriction endonuclease linker for cloning. It is also necessary to design and synthesize a sequence of a repeat segment of the recombinant protein A gene by chemical synthesis, which has a length of 168 bp and AccI restriction sites at both ends for constructing a sequence containing multiple repeat segments.
Embodiment 2
[0027] Embodiment 2, the construction of the pET32a vector containing a recombinant protein A gene monomer
[0028] Cut out the above-mentioned recombinant protein A gene monomer with restriction endonucleases NcoI and BamHI, connect with the vector pET32a digested with NcoI and BamHI, transform Escherichia coli, screen the transformant with ampicillin resistance, and extract the plasmid , After identification by enzyme digestion, it was proved that the recombinant protein A gene monomer had been cloned into pET32a.
Embodiment 3
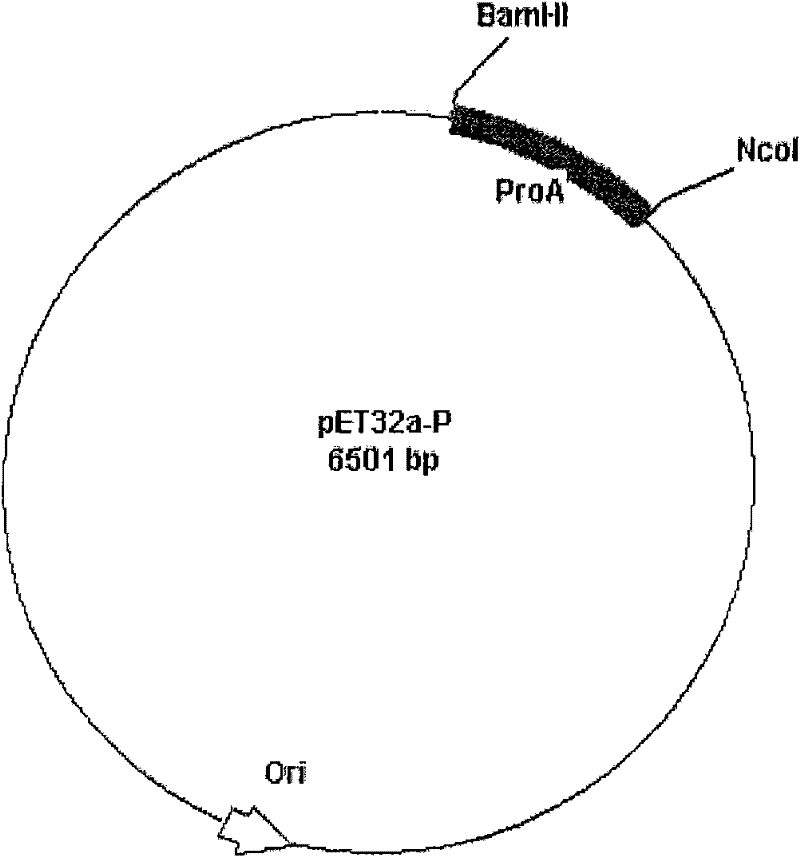
[0029] Embodiment 3, the construction of the pET32a-P vector containing recombinant protein A gene
[0030] The above pET32a vector containing a recombinant protein A gene monomer and the recombinant protein A gene monomer were digested with AccI, the corresponding fragments were recovered and ligated, transformed into Escherichia coli, and the transformants with ampicillin resistance were screened, extracted from the plasmid, and enzymatically The pET32a-P vector of recombinant protein A containing 3 protein A gene monomers was obtained by cleavage screening.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com