Perylene imide with high dissolvability, and synthesis method thereof
A technology of perylene imide and synthesis method, which is applied to the solubility of perylene imide, the field of perylene imide and its synthesis, to achieve the effect of reducing lattice energy, improving solubility and reducing interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] Production method of the present invention comprises the steps:
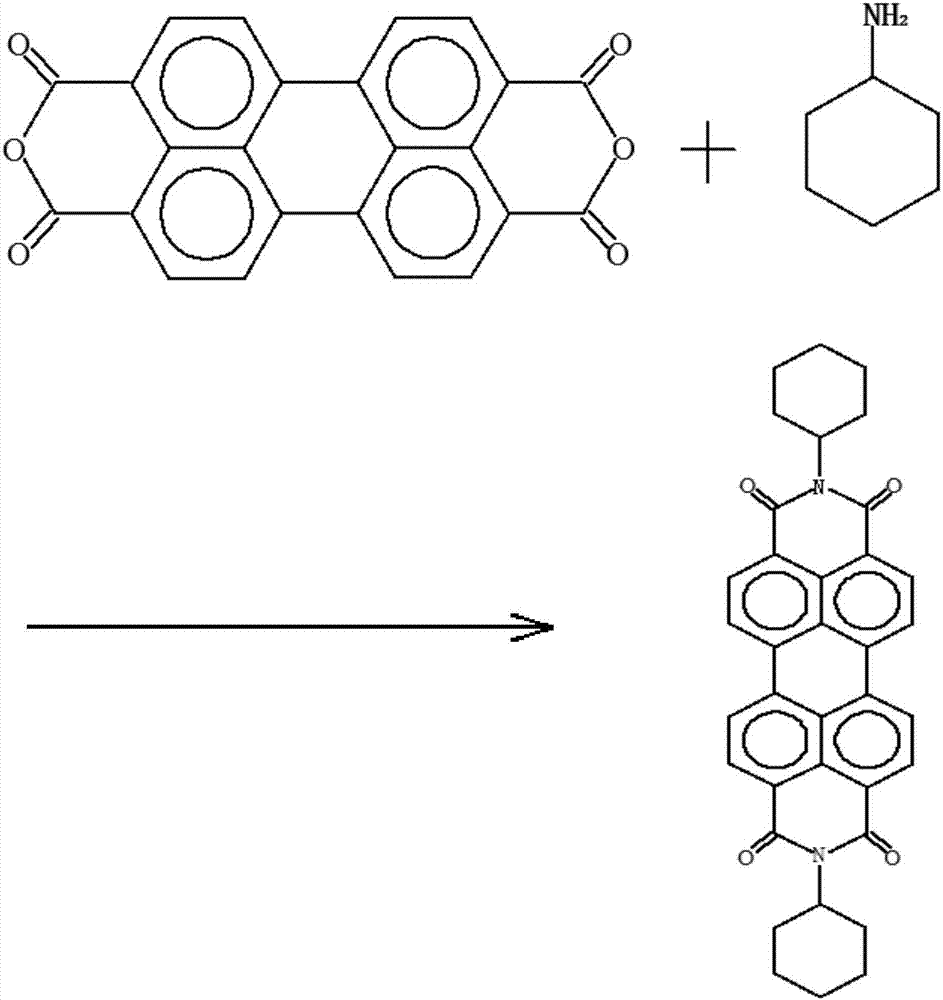
[0045] a1. Use 3,4,9,10-perylenetetracarboxylic dianhydride as raw material and cyclohexylamine under the catalyst, heat under reflux while stirring, react for about 40 hours, evaporate cyclohexylamine under reduced pressure, filter with suction, and dry to obtain N ,N'-bicyclohexyl-3,4,9,10-perylenediimide;
[0046] a2. Add dichloromethane and liquid bromine to the N,N'-bicyclohexyl-3,4,9,10-perylene diimide obtained from a1, and react after completing the reaction and cooling to room temperature, then distill off the remaining bromine and dichloromethane to obtain 1-bromo-N,N'-dicyclohexyl-3,4,9,10-perylenediimide;
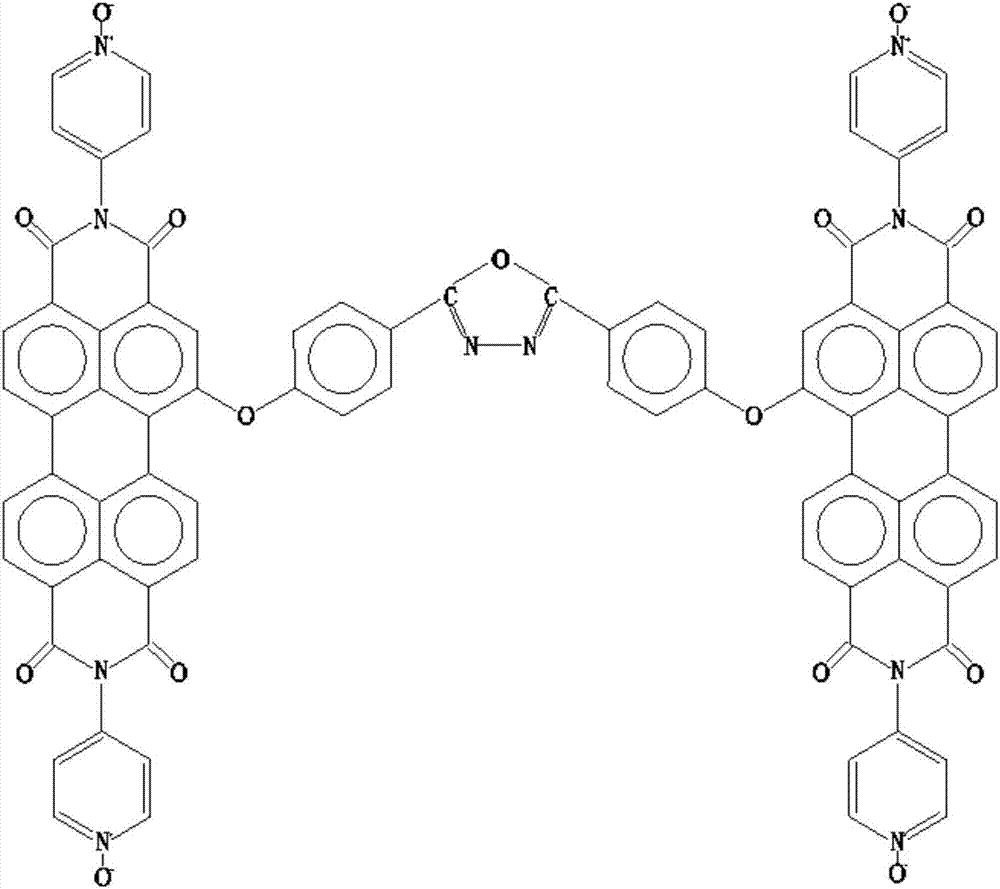
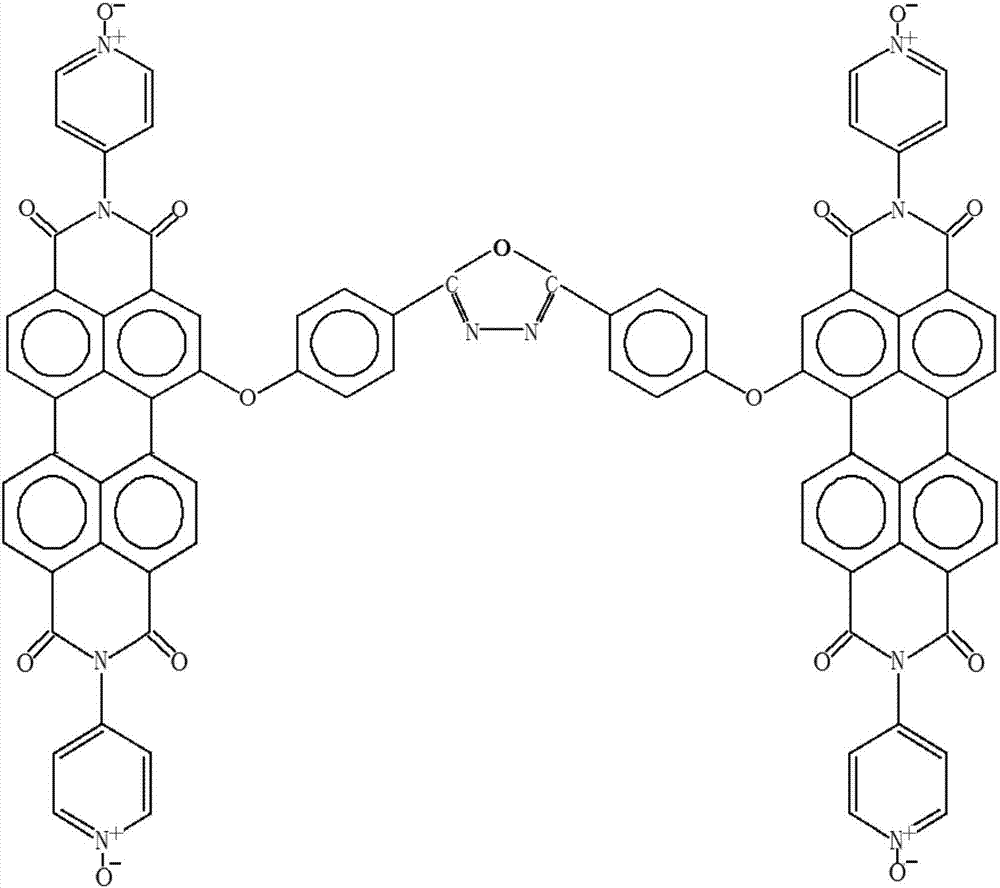
[0047] a3. The 1-bromo-3,4,9,10-perylenediimide obtained from a2 is reacted with hydroxybenzoic acid, under the action of catalyst anhydrous potassium carbonate and a polar solvent, the reaction is obtained by heating in an oil bath After the system, pour the reaction solution into deioni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com