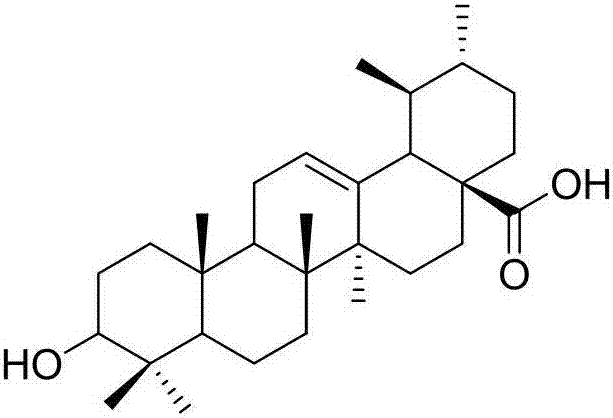
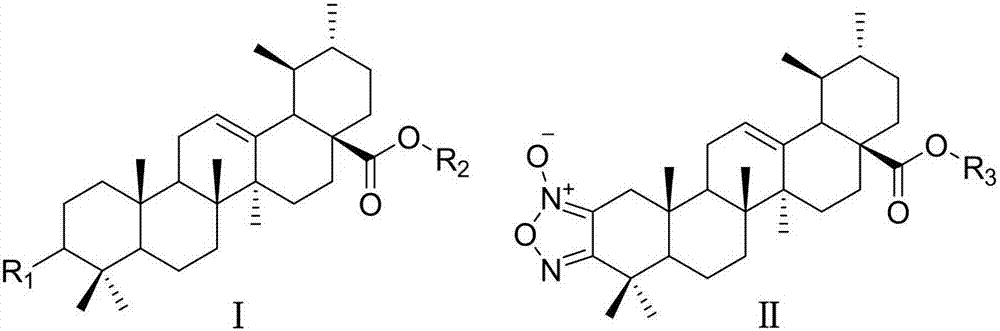
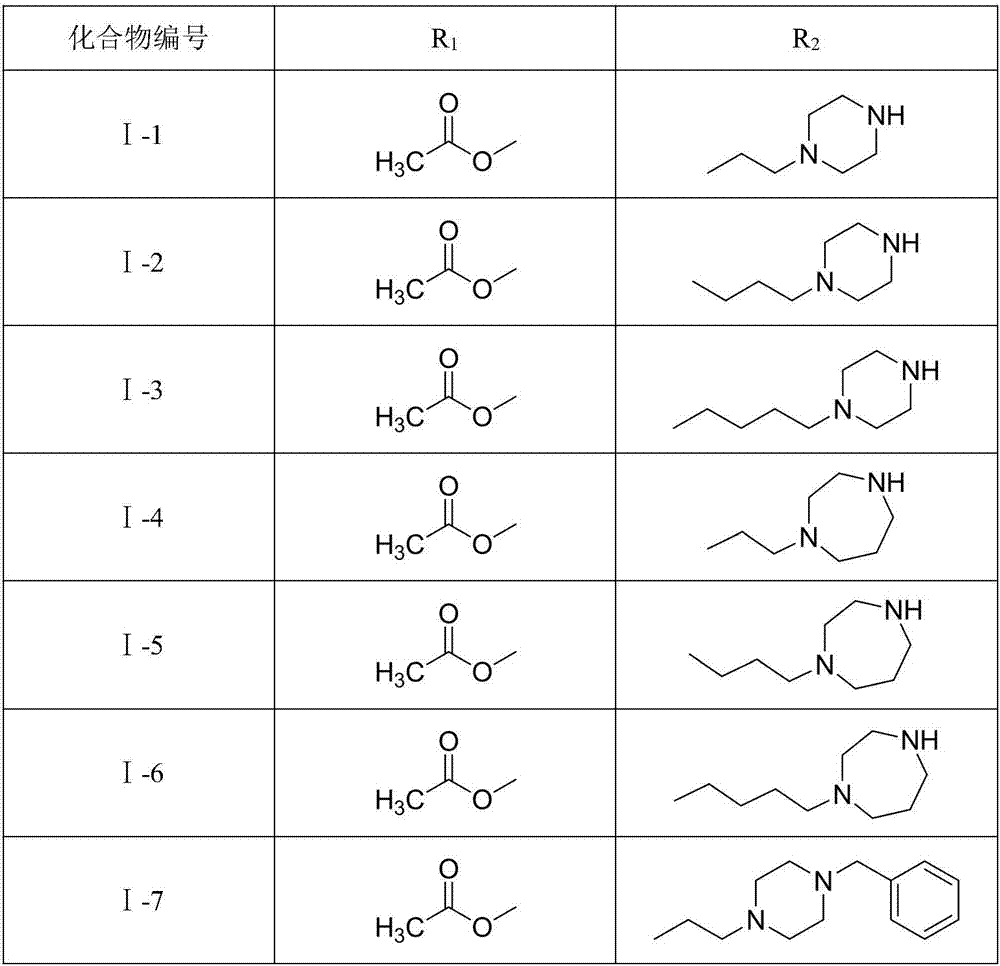
Ursolic acid derivative with antitumor activity and preparation method thereof
A technology of anti-tumor activity and ursolic acid, which is applied in the direction of anti-tumor drugs, organic chemistry, drug combination, etc., can solve the problem of low reaction yield, achieve high yield, suitable for clinical application, and significant anti-proliferation ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of compound Ⅰ-1:
[0039] Add UA (2.0g, 4.38mmol) into a 50mL eggplant-shaped bottle, dissolve it in 15mL of pyridine, control the temperature at 0°C to 10°C, slowly add acetic anhydride (1.0mL, 10.58mmol) dropwise, and stir the reaction at room temperature after the dropwise addition 14h, TLC (cyclohexane / acetone=3:1) detection showed that the reaction was complete. The reaction solution was poured into 60 mL of ice water, solids were precipitated, filtered with suction, and the filter cake was dissolved in 100 mL of ethyl acetate, washed with 1 mol / L hydrochloric acid, distilled water, and saturated aqueous sodium chloride solution, and dried overnight over anhydrous sodium sulfate. Concentrate under reduced pressure and dry in vacuo to obtain 2.02 g of 3-acetoxy-UA (2), with a yield of 92.5%.
[0040] Add compound 2 (1.16g, 2.33mmol) to a 50mL eggplant-shaped bottle, dissolve it in 15mL DMF, add 2-chloroethanol (0.58g, 7.20mmol), potassium carbonate (0.49g...
Embodiment 2
[0046] Synthesis of compound Ⅰ-4:
[0047] Referring to the synthesis method of compound I-1 in Example 1, but substituting homopiperazine for anhydrous piperazine, a yellow-white powder was obtained with a total yield of 70.2%. mp: 76.5-78.0°C.
[0048] 1 H NMR (400MHz, CDCl 3 )δ5.22(1H,s,H-12),4.53–4.44(1H,m,H-3),4.16(2H,s,CH 2 ),3.38(4H,s,H in homopiperazine),3.20(2H,s,CH 2 ), 2.97 (4H, d, J=24.4Hz, H inhomopiperazine), 2.04 (3H, s, CH 3 ),1.06(3H,s,CH 3 ),0.94(6H,d,J=3.8Hz,2×CH 3 ),0.85(9H,d,J=4.6Hz,3×CH 3 ),0.72(3H,s,CH 3 ).
[0049] HR-MS: calcd for C 39 h 65 N 2 o 4 [M+H] + 625.49388; found 625.49126.
Embodiment 3
[0051] Synthesis of compound Ⅰ-8:
[0052] Add compound Ⅰ-1 (60mg, 0.098mmol) to a 10mL chicken heart bottle, dissolve it in 2mL of DMF, add potassium carbonate (17mg, 0.123mmol), 4-fluorobenzyl bromide (0.02mL, 0.161mmol), and react in an oil bath at 60°C After 30 min, TLC (dichloromethane / methanol=20:1) showed that the reaction was complete. After natural cooling, it was extracted with 50 mL of ethyl acetate and 30 mL of water, the organic phase was washed twice with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and purified by preparative TLC (dichloromethane / methanol=20:1) to obtain the product 29.8 mg (I-8), yellow-white powder, yield 42.3%, mp: 140.0-141.8 °C.
[0053] 1 H NMR (400MHz, CDCl 3 )δ7.76–7.58(2H,m,ArH),7.12(2H,t,J=8.2Hz,ArH),5.16(1H,s,H-12),4.51–4.43(1H,m,H-3 ),4.08(2H,s,CH 2 ),2.73–3.90(10H,m,CH 2 and 4×CH 2 inpiperazine), 2.09 (1H, d, J=11.3Hz, H-18), 2.04 (3H, s, CH 3 ),1.04(3H,d,J=7.4Hz,CH 3 ),0.92(6H,d,J=10.4Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com