An ultrafast preparation method for n, ti3+ co-doped porous tio2 nanosheets
A nanosheet and co-doping technology, applied in the direction of nanotechnology, titanium oxide/hydroxide, titanium dioxide, etc., can solve the problems of high temperature and long time required, and achieve short time consumption and fast deflagration reaction rate , The effect of simple preparation method conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1 g TiO at room temperature 2 Nanosheets and 0.2g NaN 3 Add the powder into 5mL deionized water, stir it with magnetic force to make it evenly mixed, pour it slowly into an ark filled with liquid nitrogen and freeze it quickly, and place it in a closed explosive device after freeze-drying; use electric ignition or heating to trigger Deflagration reaction, collect the obtained product after the reaction, wash it repeatedly with deionized water, and get N, Ti 3+ Co-doped porous TiO 2 Nanosheets.
Embodiment 2
[0040] 1 g TiO at room temperature 2 Nanosheets and 0.1 g NaN 3 The powder was added to 5 mL of deionized water, stirred evenly by magnetic force, slowly poured into an ark filled with liquid nitrogen and quickly frozen, and placed in a closed explosive device after freeze-drying; other steps were the same as in Example 1.
Embodiment 3
[0042] 1 g TiO at room temperature 2 Nanosheets and 0.3g NaN 3 The powder was added to 5 mL of deionized water, stirred evenly by magnetic force, slowly poured into an ark filled with liquid nitrogen and quickly frozen, and placed in a closed explosive device after freeze-drying; other steps were the same as in Example 1.
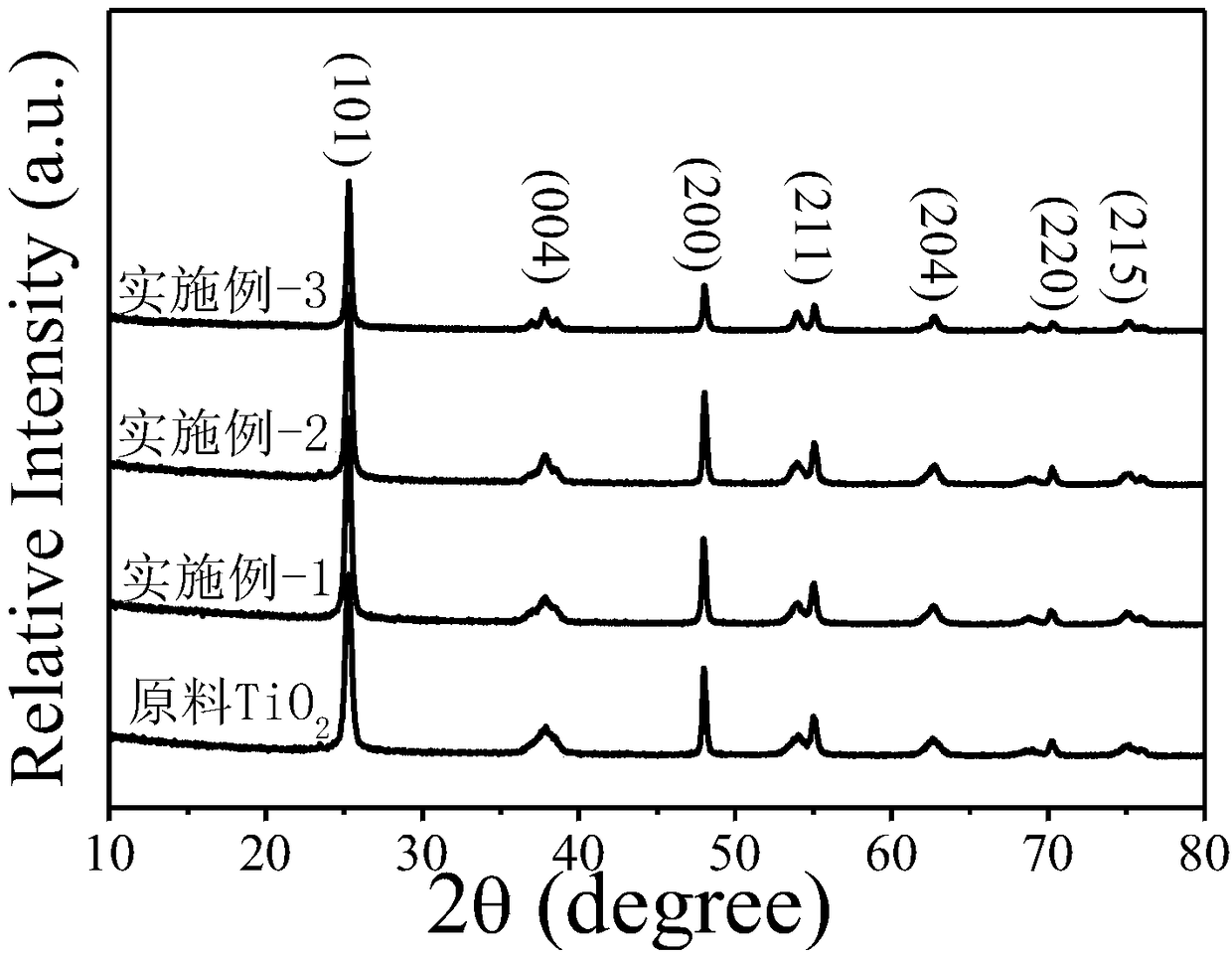
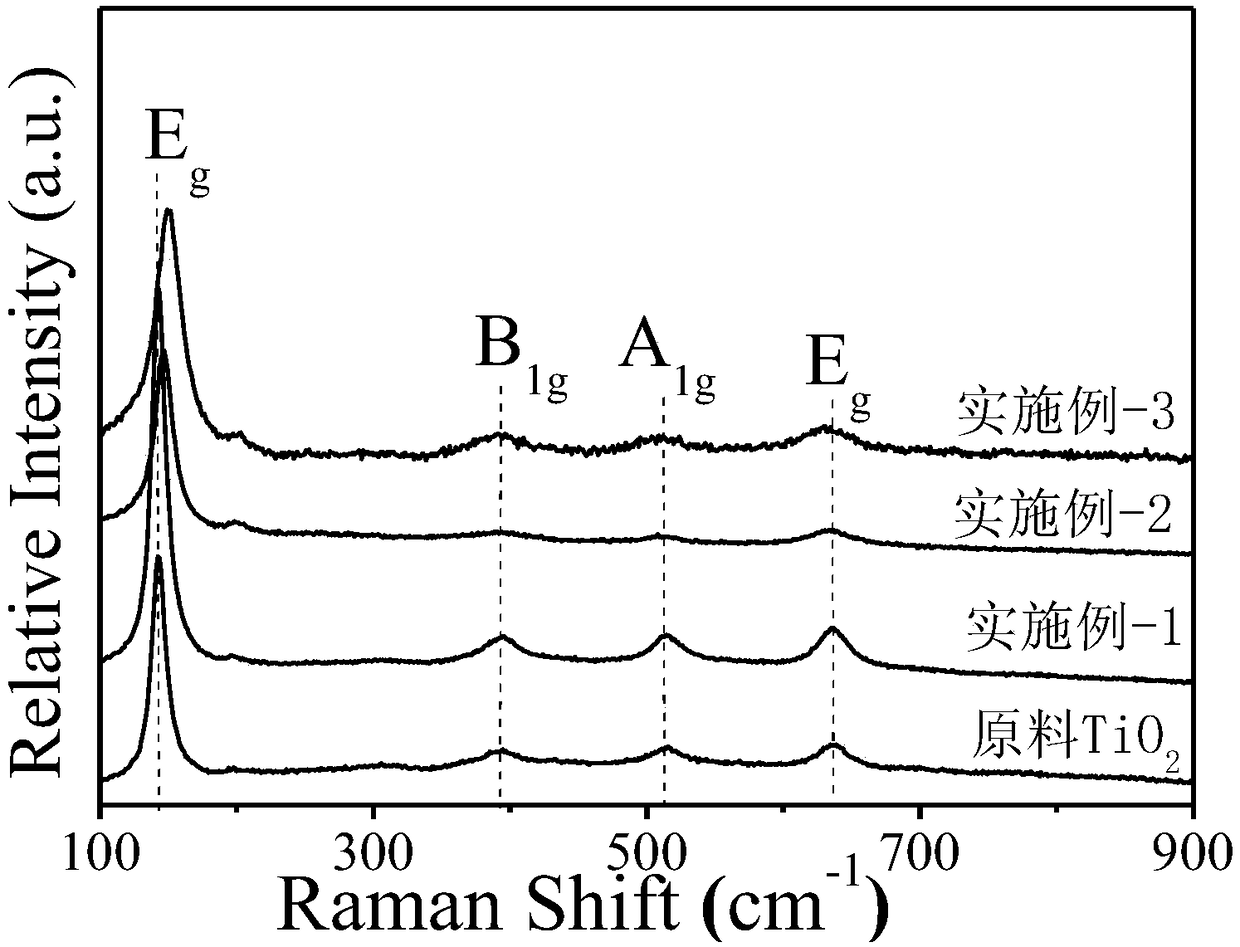
[0043] The N, Ti that embodiment 1~3 prepares 3+ Co-doped porous TiO 2 Nanosheets and Raw TiO 2 Nanosheets were analyzed and compared. The result is as Figure 2-8 shown.
[0044] Among them, from figure 2 It can be seen in the XRD pattern of NaN 3 After the deflagration treatment, the diffraction peaks of the samples prepared in Examples 1-3 did not change significantly, and they were all anatase phase TiO 2 (JCPDS Card 21-1272), stating NaN 3 Deflagration treatment did not produce impurities.
[0045] From image 3 It can be seen in the Raman spectrum that the N, Ti prepared in Examples 1-3 3+ Co-doped porous TiO 2 The Raman peak of the nano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com