Formula of serum free medium for human pluripotent stem cells
A technology of pluripotent stem cells and serum-free medium, applied in the direction of non-embryo pluripotent stem cells, artificially induced pluripotent cells, cell culture active agents, etc., to achieve the effect of wide application and large market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
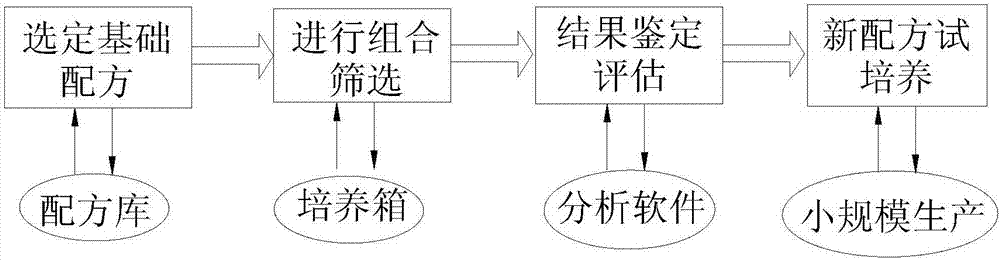
[0035] A formula for serum-free medium for human pluripotent stem cells, including the following raw materials: inorganic salt components, organic components, amino acids and amino acid salts, energy substances and metabolic intermediates, vitamins and antioxidants, proteins and polypeptides, trace elements and chromogenic substances; human pluripotent stem cells were expanded and cultured according to the above method.
[0036] Expansion culture of human pluripotent stem cells: add freshly prepared bFGF (ie: basic fibroblast growth factor, bFGF 2 mg per liter of medium), β-mercaptoethanol (per liter of culture medium) to the prepared medium solution Add β-mercaptoethanol 7μl); maintain the undifferentiated state of human pluripotent stem cells, and make pluripotent stem cells self-renew and proliferate through expansion culture, so as to increase the number of cells, expand the reserve, and carry out subsequent subpackaging and preservation The purpose; with the current marke...
Embodiment 2
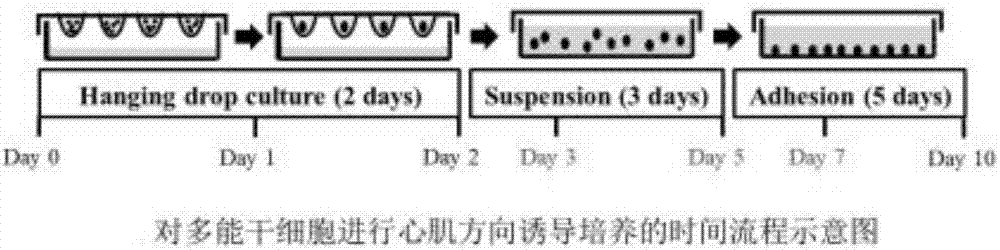
[0038] see Figure 2-3 : A formula for serum-free medium for human pluripotent stem cells, including the following raw materials: inorganic salt components, organic components, amino acids and amino acid salts, energy substances and metabolic intermediates, vitamins and antioxidants, proteins and peptides, trace amounts Elements and chromogenic substances; myocardial induction culture was carried out according to the above method, and the culture process was divided into: hanging drop culture for 3 days + suspension culture for 2 days + adherent culture for 5 days, and vitamin C was added at the same time (128.05 mg of ascorbicacid was added to each liter of culture solution) ).
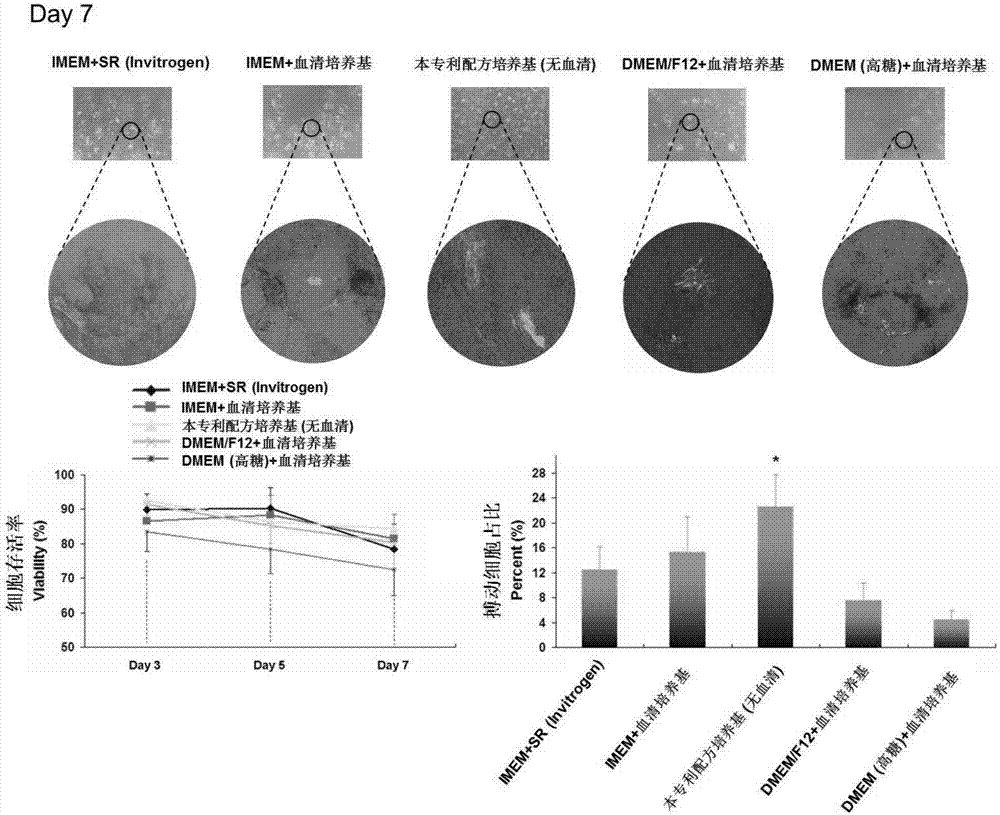
[0039] Cardiac induction culture: Human pluripotent stem cell line IMR90-1 (Wicell Research Institute, Madison, WI, USA) was used to induce the reporter gene MHC-eGFP (myosin heavy chain promoter-driven After the initiation of the induction culture, once the stem cells differentiate into the myocard...
Embodiment 3
[0041] see Figure 4-5 : A formula for serum-free medium for human pluripotent stem cells, including the following raw materials: inorganic salt components, organic components, amino acids and amino acid salts, energy substances and metabolic intermediates, vitamins and antioxidants, proteins and peptides, trace amounts Elements and chromogenic substances; Neural induction was carried out according to the above method: HS-5 cell line was used as a feeder layer in the prepared medium solution, and NT-3 was added at the same time (1342 mg of NT-3 was added per liter of medium).
[0042] Neural induction culture: Human pluripotent stem cell line iPS-1 (Wicell Research Institute, Madison, WI, USA) was co-cultured with human bone marrow stromal cell line HS-5 ( Figure 4 ), to achieve the purpose of inducing and culturing pluripotent stem cells toward nerve cells; the current market similar products to be compared include DMEM / F12+10% fetal bovine serum, Knockout DMEM+10% SR (Invit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com