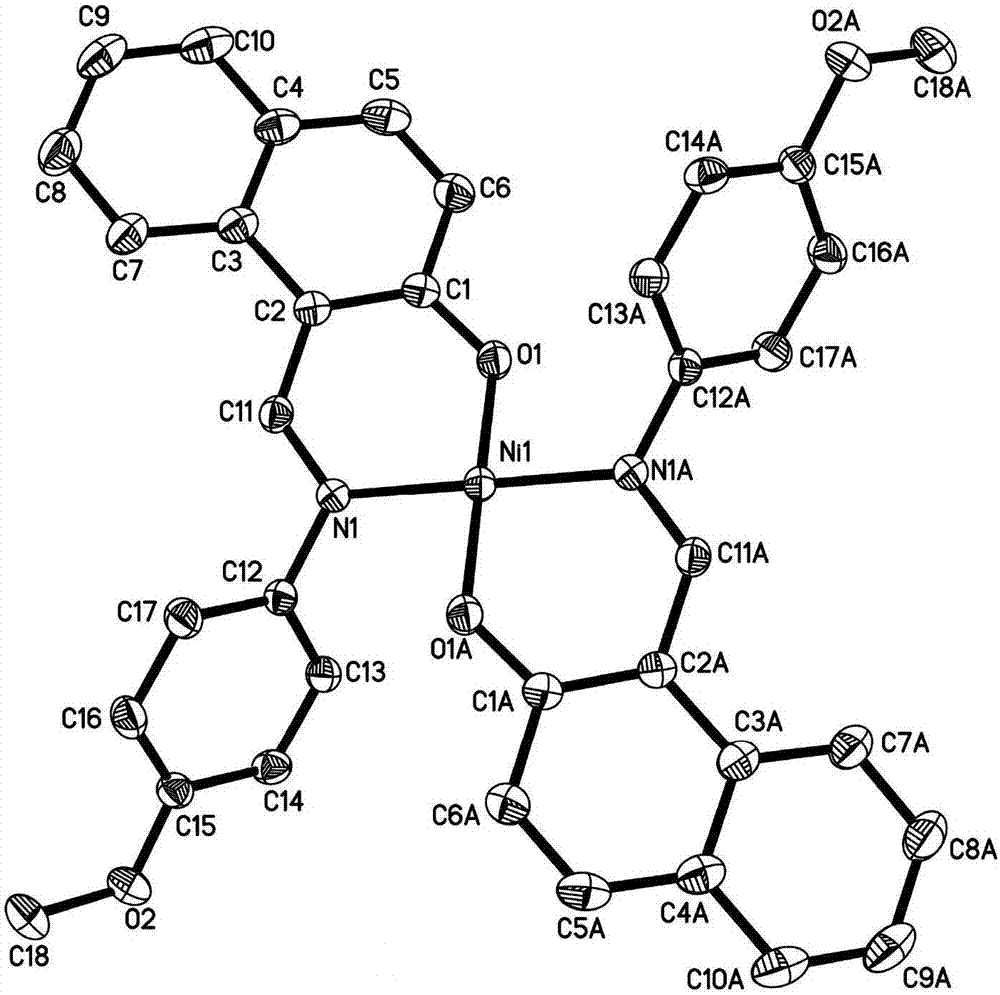
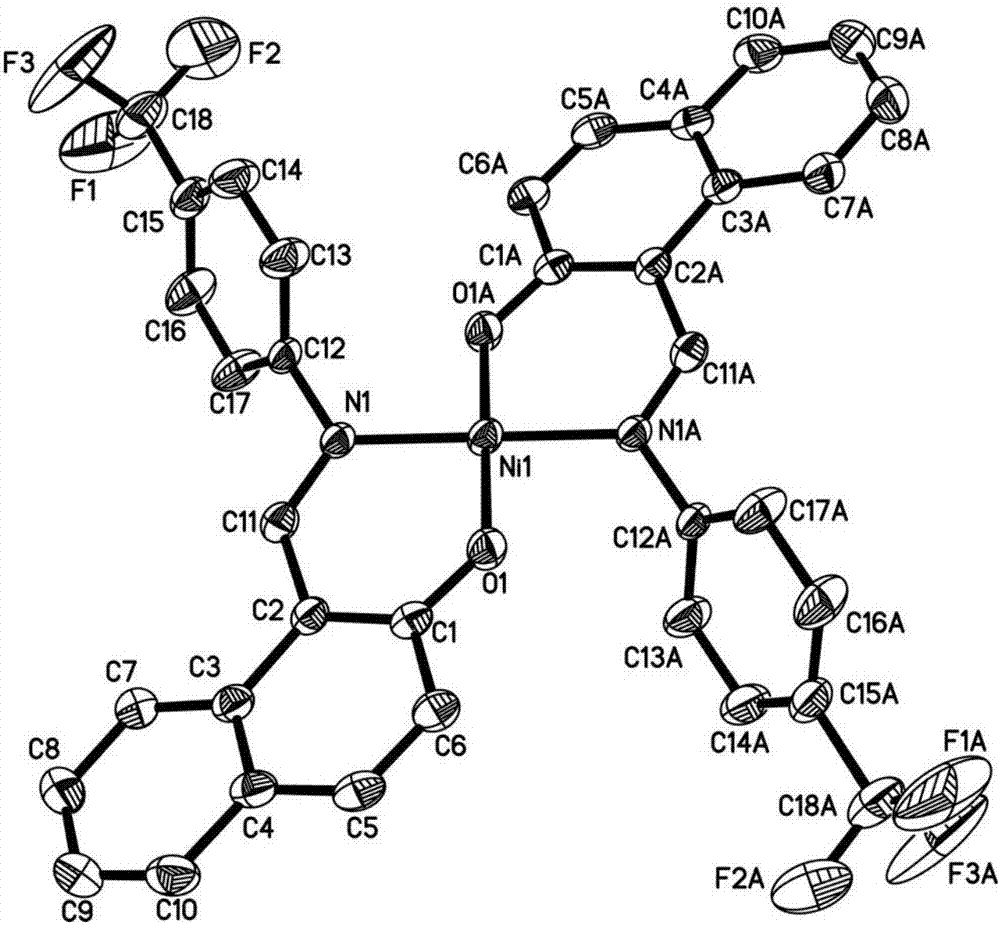
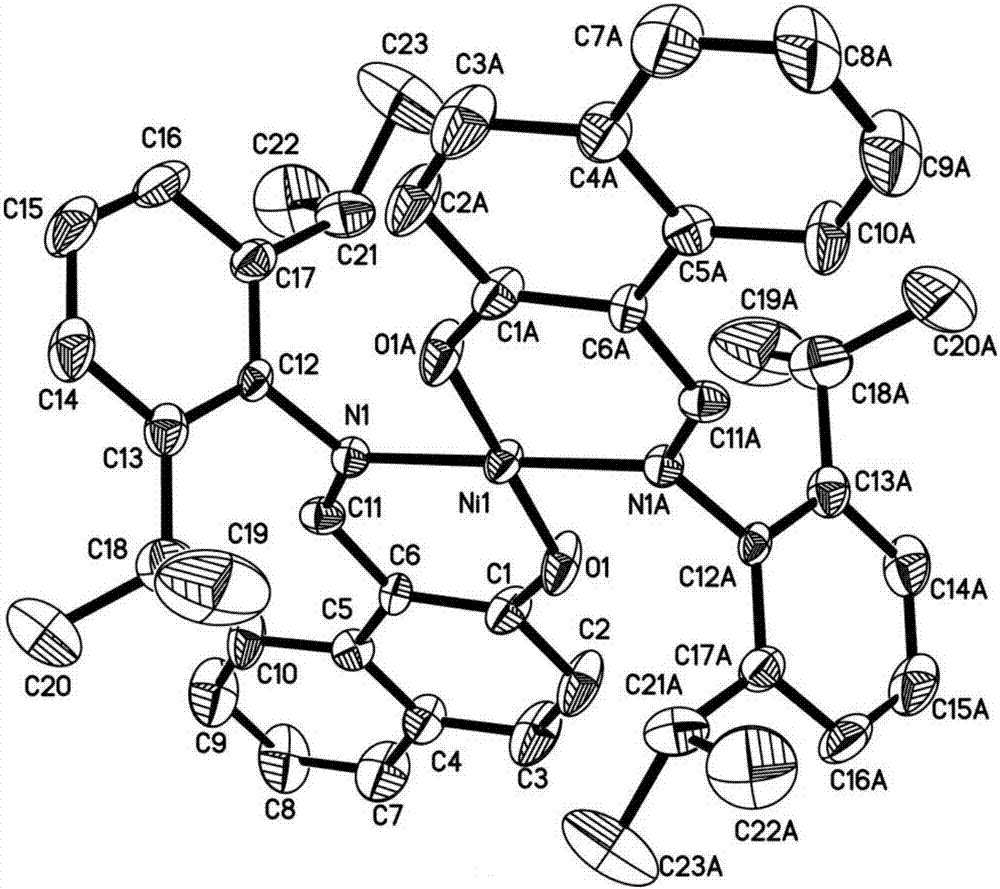
N,O-coordinated bivalent nickel complex with binaphthol structure and preparation method thereof
A technology of divalent nickel and complexes, which is applied in the preparation of organic compounds, imino compounds, carboxylic acid nitriles, etc., can solve the problems of low yield and complicated method, and achieve high yield, high catalytic activity, stable The effect of physicochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Synthesis of Schiff base ligand L1
[0025] Dissolve 12mmol of 2-hydroxy-1-naphthaldehyde and 10mmol of aromatic amine (R=H) in 50mL of methanol, add two drops of glacial acetic acid dropwise, heat up to 60°C for 4 hours, after the reaction, cool to room temperature and put it in the refrigerator Overnight, a solid was formed, which was filtered, washed quickly with a small amount of refrigerated methanol, and dried to obtain the Schiff base ligand L1 with a yield of 92%.
[0026] Elemental Analysis: C 17 h 13 NO: C 82.57, H 5.30, N 5.66, found: C 82.68, H 5.35, N 5.47.
Embodiment 2
[0027] Example 2: Synthesis of Schiff base ligand L2
[0028] 12mmol 2-hydroxyl-1-naphthaldehyde and 10mmol aromatic amine (R=4-OCH 3 ) was dissolved in 50mL of methanol, two drops of glacial acetic acid were added dropwise, and the temperature was raised to 60°C to react for 4 hours. Schiff base ligand L2, yield 88%.
[0029] Elemental Analysis: C 18 h 15 NO 2 : C 77.96, H 5.45, N 5.05, found: C 77.79, H 5.38, N 5.23.
Embodiment 3
[0030] Example 3: Synthesis of Schiff base ligand L3
[0031] Dissolve 12mmol of 2-hydroxy-1-naphthaldehyde and 10mmol of aromatic amine (R=CN) in 50mL of methanol, add two drops of glacial acetic acid dropwise, raise the temperature to 60°C for 4 hours, after the reaction, cool to room temperature and put it in the refrigerator Overnight, a solid was formed, which was filtered, washed quickly with a small amount of refrigerated methanol, and dried to obtain the Schiff base ligand L3 with a yield of 85%.
[0032] Elemental Analysis: C 18 h 12 N 2 O: C 79.39, H 4.44, N 10.29, found: C 79.45, H 4.49, N 10.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com