Preparation method of composite membrane with catalytic function for positive and negative electrodes used for all-vanadium redox flow batteries
An all-vanadium redox flow battery and composite membrane technology, applied in the field of composite membrane preparation, can solve problems such as difficulty in obtaining a proton conducting membrane with comprehensive properties, achieve good vanadium resistance performance, reduce the cost of energy storage systems, and improve vanadium battery performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In this embodiment, the specific steps are as follows:
[0028] 1. Dissolve 0.2g bismuth nitrate in 10mL N,N-dimethylformamide (DMF), stir and dissolve, and use it for the catalytic functional layer on the negative electrode side; dissolve 0.15g tungsten phosphate in 10mL N,N-dimethylformamide (DMF) Formamide (DMF), stirred and dissolved, used for the catalytic functional layer on the positive electrode side.
[0029] 2. Dissolve 4g of perfluorosulfonic acid resin in N,N-dimethylformamide (DMF), heat and dissolve in an autoclave to prepare a 6% perfluorosulfonic acid resin solution by mass, and heat to dissolve The temperature condition was 220°C.
[0030] 3. Ultrasonic treatment of the solution obtained in step (2) for 1 h to remove air bubbles and impurities.
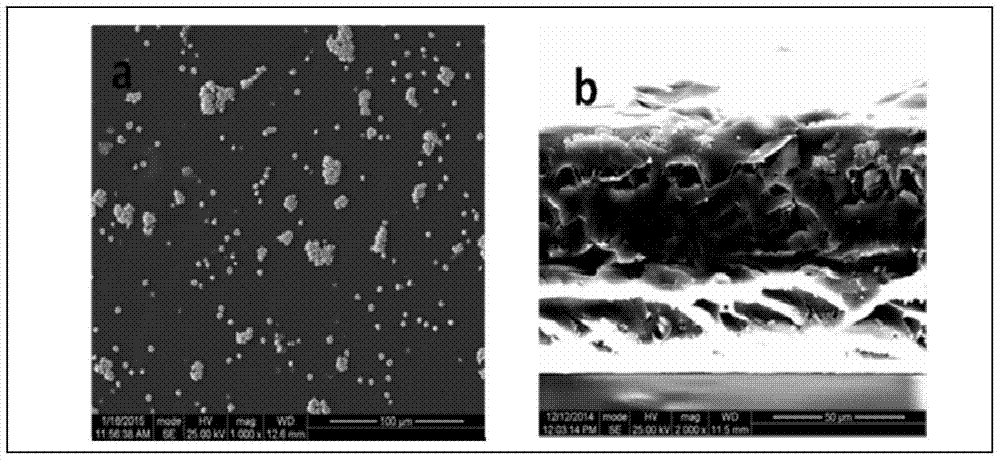
[0031] 4. Using the solution casting method, cast 50 mL of the perfluorosulfonic acid resin solution in step (3) on a glass plate, dry at 120° C. for 2 hours and volatilize to form a film. The thickness of th...
Embodiment 2
[0037] The difference from Example 1 is:
[0038] 1. Dissolve 0.4g bismuth nitrate in 10mL N,N-dimethylformamide (DMF), stir and dissolve, and use it for the catalytic functional layer on the negative electrode side; dissolve 0.3g tungsten phosphate in 10mL N,N-dimethylformamide (DMF) Formamide (DMF), stirred and dissolved, used for the catalytic functional layer on the positive electrode side.
[0039] 2. The remaining steps are the same as in Example 1.
[0040] In this example, the thickness of the obtained composite film is 70 μm, and the interfaces in the composite film are in good contact, but the uniformity and flatness of the film surface are not very good.
Embodiment 3
[0042] The difference from Example 1 is:
[0043] 1. Dissolve 0.1g bismuth nitrate in 10mL N,N-dimethylformamide (DMF), stir and dissolve, and use it for the catalytic functional layer on the negative electrode side; dissolve 0.75g tungsten phosphate in 10mL N,N-dimethylformamide (DMF) Formamide (DMF), stirred and dissolved, used for the catalytic functional layer on the positive electrode side.
[0044] 2. The remaining steps are the same as in Example 1.
[0045] In this embodiment, the obtained composite film has a thickness of 55 μm, and the interfaces in the composite film are in good contact without any separation phenomenon.
[0046] The relevant performance data of the present embodiment are as follows:
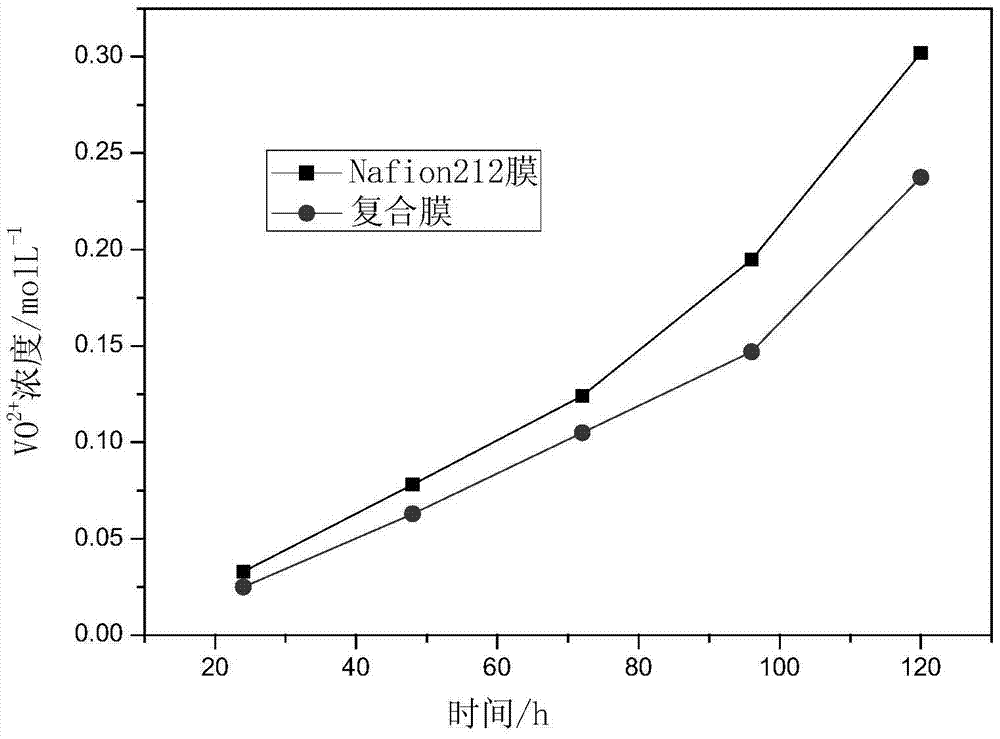
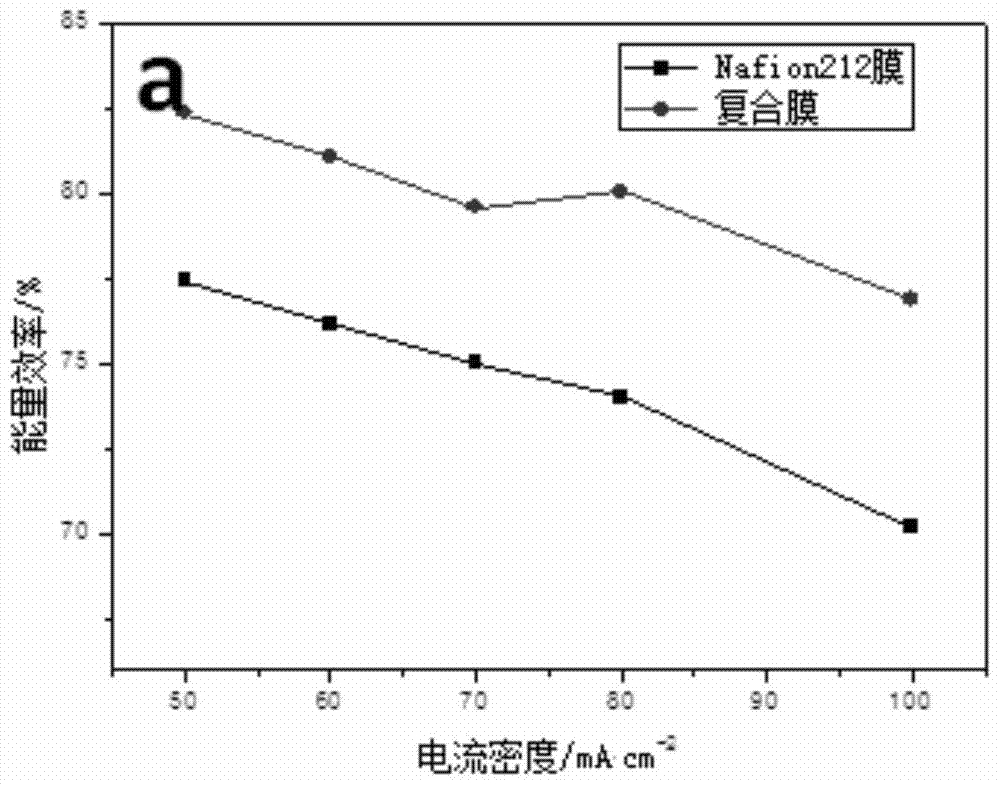
[0047] It is measured at room temperature that the coulombic efficiency and energy efficiency of the composite film in the all-vanadium redox flow battery are lower than the data in Example 1 by about 2%. The analysis reason is that the catalytic layer is thinner and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com