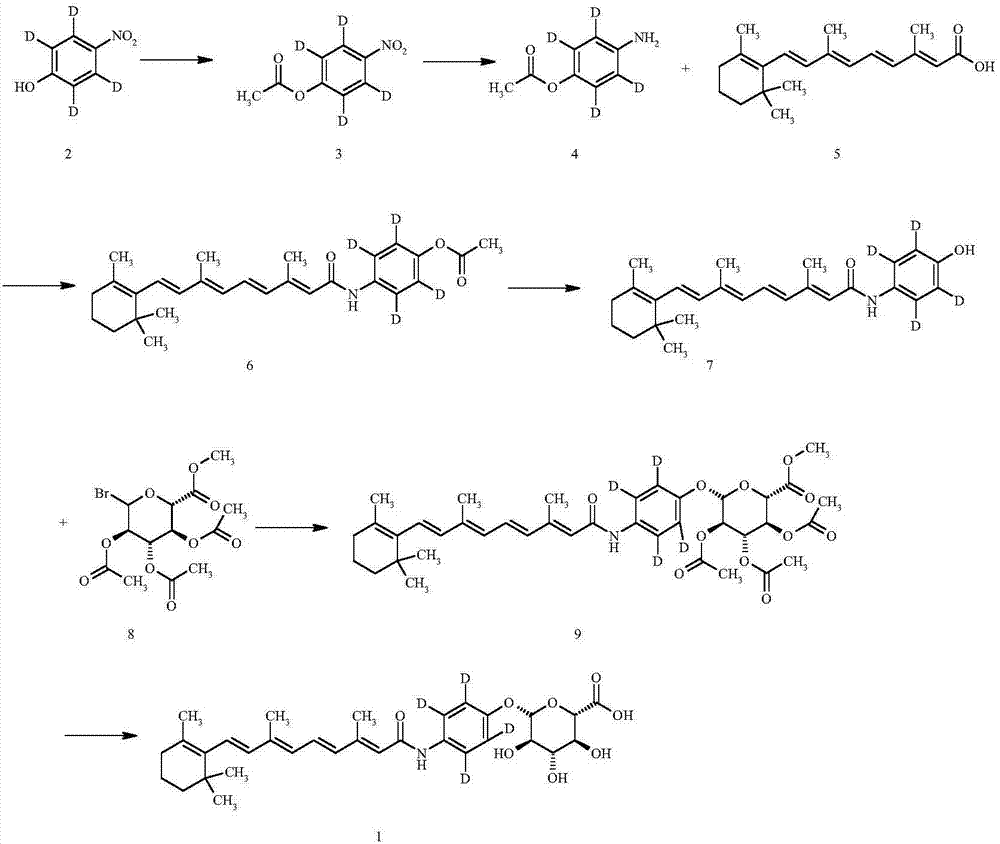
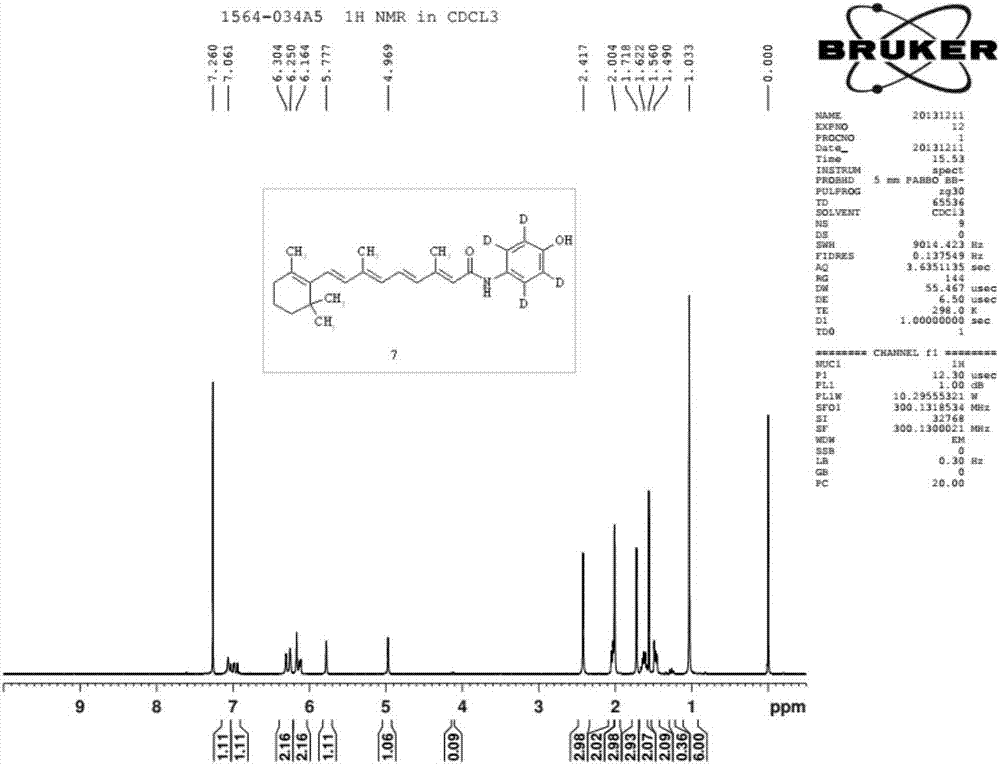
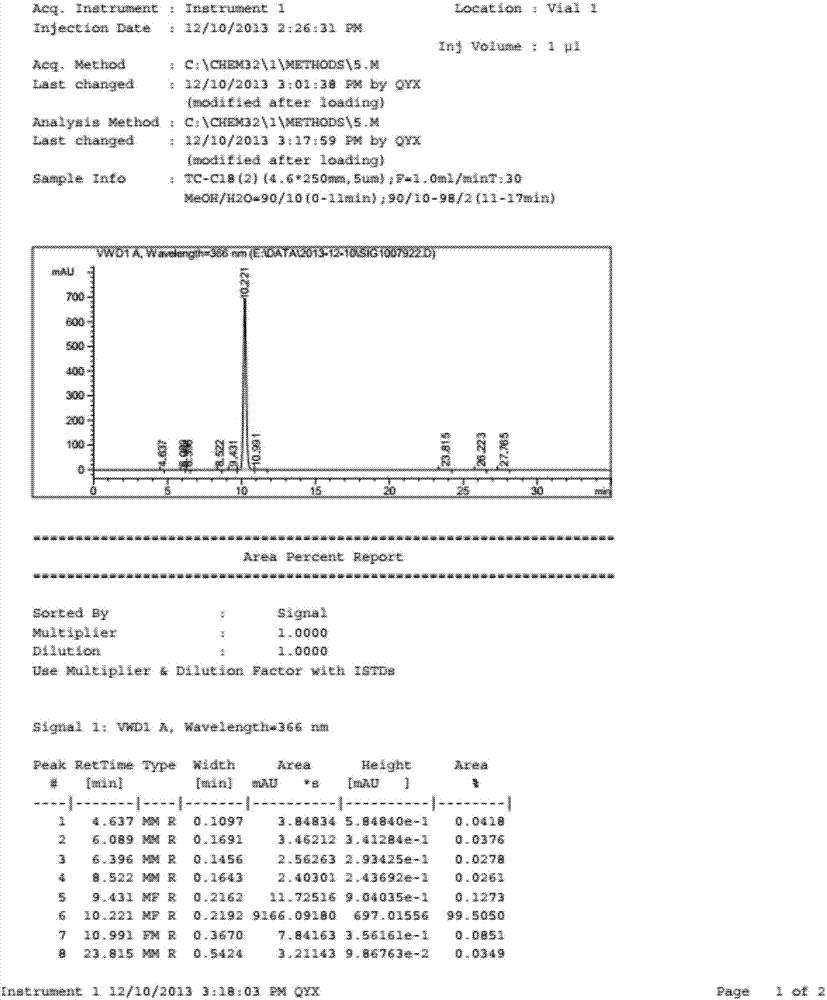
Synthesizing method of deuterium-labeled glucuronide fenretinide
A technology of retinoyl glucuronide and synthesis method, which is applied in the field of synthesis of deuterium-labeled retinoyl glucuronide, and can solve the problem of retinoyl glucuronide that has not been found. To solve problems such as the synthesis method of acylphenamides, achieve the effects of excellent antitumor activity, simple and easy-to-obtain reagents, and reasonable synthesis process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of Compound 3:
[0044]
[0045] To a 100ml round bottom flask, add 4-nitrophenol-D in sequence 4 (2.25g, 15.7mmol), 16.9ml of dry pyridine and 3.94ml of acetic anhydride, the reaction was heated to an oil bath at 90°C and stirred for 2 hours, and the reaction solution was brown. After TLC showed that the raw materials had reacted completely, cooled to room temperature (18 degrees), spin-dried pyridine, added 20ml of water, stirred, a large amount of solids were precipitated, filtered with suction, washed with an appropriate amount of water, drained, and dried overnight in a vacuum dryer to obtain Product Compound 3 (2.88 g, off-white solid). Yield 98.95%.
[0046] Preparation of Compound 4:
[0047]
[0048] In a 100ml round bottom flask, add the prepared compound 3 (2.88g, 15.5mmol) and 43.2ml methanol successively. The raw materials are not easily soluble. Weigh and add 0.43g Pd / C successively under nitrogen protection, and react with hydrogen ga...
Embodiment 2
[0064] Preparation of compound 3:
[0065]
[0066] To a 100ml round bottom flask, add 4-nitrophenol-D in sequence 4 (3g, 20.9mmol), 30ml of dry pyridine and 6ml of acetic anhydride, the reaction temperature was raised to 100°C in an oil bath and stirred for 1 hour, the reaction solution was brown. After TLC showed that the raw materials had reacted completely, cooled to room temperature (20 degrees), spin-dried pyridine, added 50ml of water, stirred, a large amount of solids were precipitated, filtered with suction, washed with an appropriate amount of water, drained, and dried overnight in a vacuum dryer to obtain Product Compound 3 (3.0 g, off-white solid). Yield 77.30%.
[0067] Preparation of compound 4:
[0068]
[0069] In a 150ml round bottom flask, add the prepared compound 3 (3g, 16.2mmol) successively, 60ml methanol, the raw material is not easily soluble, weigh and add 0.6g Pd / C successively under the protection of nitrogen, react hydrogen, at room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com