Multi-drug combined rehabilitation granule for type 2 diabetes and application thereof
A technology for type 2 diabetes and granules, which is applied to the multi-drug combination rehabilitation granules for type 2 diabetes and their application fields, can solve the problems of increased drug resistance, negative effects, and degeneration of autologous islet function, and achieves the effect of improving utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The mass of sorbitol is 35 grams, the mass of Span 60 is 0.1 grams, and the mass of betaine is 8 grams. After uniform mixing, the multi-drug combination rehabilitation granule for type 2 diabetes is obtained;
[0025] Pour the multi-drug combination rehabilitation granule into 500ml water at 80°C and stir until completely dissolved; the solution needs to be drunk within 15-120 minutes.
Embodiment 2
[0027] The mass of sorbitol is 35 grams, the mass of betaine is 10 grams, and the mass of Span 60 is 0.3 grams. After uniform mixing, the multi-drug combination rehabilitation granule for type 2 diabetes is obtained;
[0028] Pour the multi-drug combination rehabilitation granule into 500ml water at 80°C and stir until completely dissolved; the solution needs to be drunk within 15-120 minutes.
Embodiment 3
[0030] The quality of sorbitol is 40 grams, the quality of betaine is 10 grams, and the quality of Span 60 is 0.2 grams. After uniform mixing, the multi-drug combination rehabilitation granule for type 2 diabetes is obtained;
[0031] Pour the multi-drug combination rehabilitation granule into 500ml water at 80°C and stir until completely dissolved; the solution needs to be drunk within 15-120 minutes.
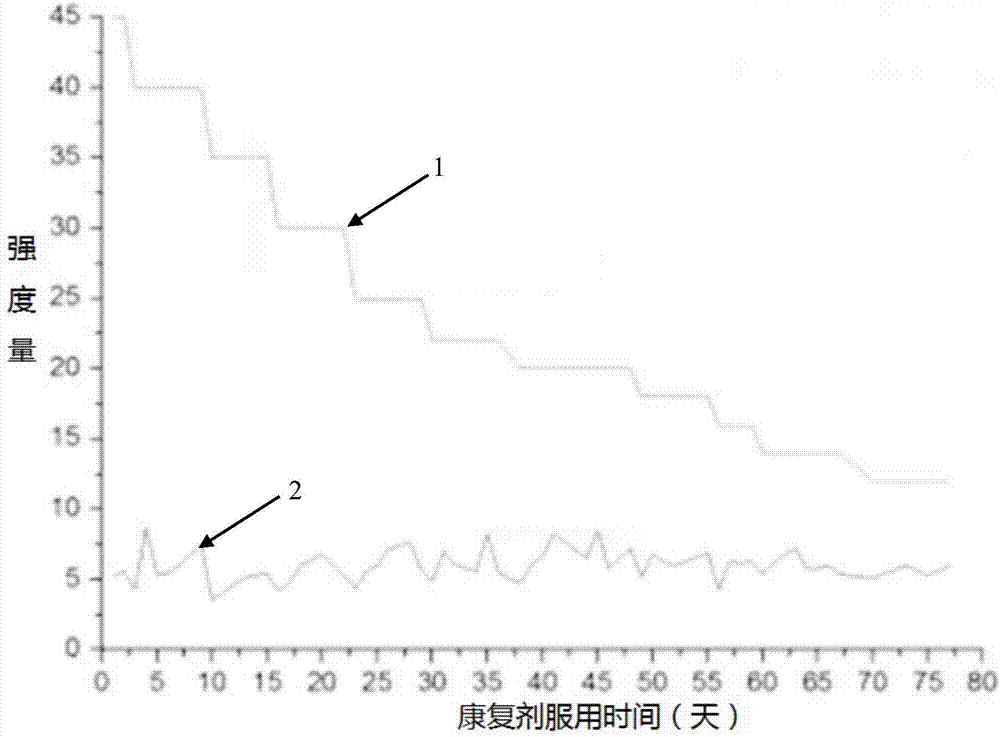
[0032] The type 2 diabetes multi-medicine rehabilitation granule of the present invention may cause diarrhea, abdominal distension, and increased upper and lower exhaust gas after taking it, which can be slowed down or prevented from occurring by prolonging the drug taking time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com