Phosphinoxy red/orange thermally excited delayed fluorescent material, synthesis method and application
A technology of delayed fluorescence and phosphine red, which is applied in luminescent materials, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., can solve the problems of concentration quenching, fast decay, and low efficiency of light-emitting devices , to improve efficiency, inhibit intermolecular interactions, and reduce quenching effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
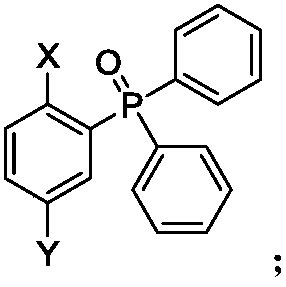
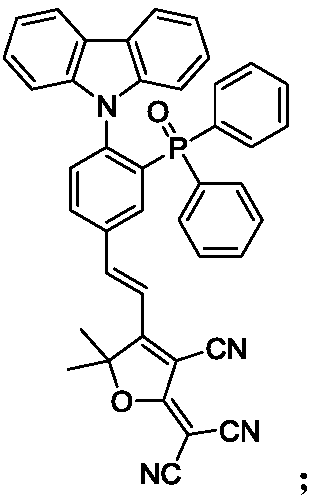
[0057] Specific embodiment 1: In this embodiment, the structural formula of phosphine red / orange photothermal excitation delay fluorescent material is as follows:
[0058] When X is carbazolyl, Y is , its structural formula is:
[0059] When X is 3,6-di-tert-butylcarbazolyl, Y is , its structural formula is:
[0060] When X is carbazolyl, Y is , its structural formula is:
[0061] When X is 3,6-di-tert-butylcarbazolyl, Y is , its structural formula is:
[0062] When X is carbazolyl, Y is , its structural formula is:
[0063] When X is 3,6-di-tert-butylcarbazolyl, Y is , its structural formula is:
specific Embodiment approach 2
[0064] Specific embodiment two: the synthetic method of phosphine red / orange photothermal excitation delayed fluorescent material described in specific embodiment one, the synthetic method is as follows:
[0065] 1. Mix 2~5mmol of carbazole or 3,6-di-tert-butylcarbazole, 1mmol of 3-bromo-4-fluorobenzaldehyde, 5~20ml of dimethyl sulfoxide and 2~5mmol of potassium carbonate , stirred and reacted at 150°C for 12-24 hours, then poured into ice water, filtered with suction, dissolved the obtained solid in benzene, added 5-10 mmol of ethylene glycol, 0.1-1 mmol of p-toluenesulfonic acid, and refluxed for 3- After cooling for 12 hours, extract with water and dichloromethane, combine the organic layers, remove the organic solvent after drying, and recrystallize with absolute ethanol to obtain 9-(2-bromo-4-(1,3-dioxolane- 2-yl)phenyl)-9H-carbazole or 9-(2-bromo-4-(1,3-dioxolan-2-yl)phenyl)-3,6-di-tert-butyl-9H - carbazole;
[0066] 2. Dissolve the product synthesized in Step 1 in tet...
specific Embodiment approach 3
[0068] Specific embodiment three: the difference between this embodiment and specific embodiment two is that in step one, 3 mmol of carbazole or 3,6-di-tert-butyl carbazole, 1 mmol of 3-bromo-4-fluorobenzaldehyde, 10 ml of Dimethyl sulfoxide and 4 mmol of potassium carbonate were mixed. Others are the same as the specific implementation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com