Truncated growth differentiation factor 11 with biological activity and preparation method thereof
A technology of growth and differentiation factors and biological activity, which is applied in the field of biomedicine and can solve the problem of high cutting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
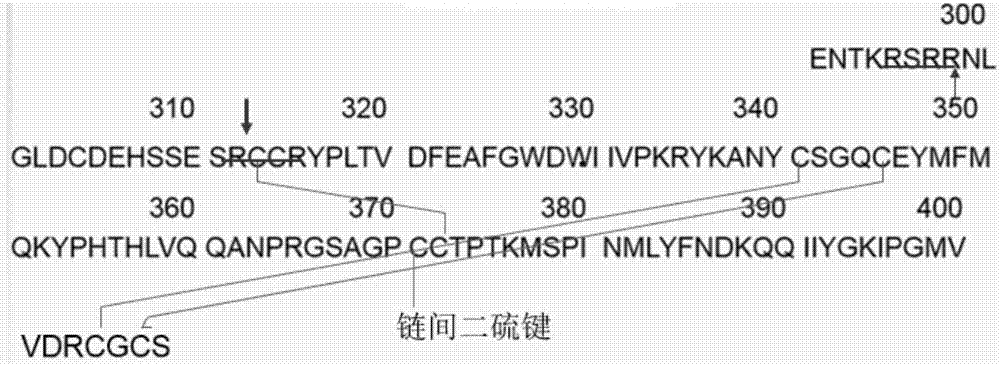
[0033] Example 1 Preparation of truncated GDF11
[0034] 1. Preparation of SUMO proteolytic enzyme (His-ULP) with His tag
[0035] (1) Synthesize the His-ULP gene, add 5'NcoI, 3'XhoI recognition sequence, the nucleotide sequence of the His-ULP gene is shown in SEQ ID NO.3, and use NcoI and XhoI double digestion;
[0036] (2) Digest the pET-28a(+)(kan) plasmid with NcoI and XhoI, connect and recombine with the DNA fragment obtained in step (1) after digestion, transform JM109, screen positive clones, and sequence to obtain the correct sequencing result Recombinant plasmid named pET-28a(+)-ULP;
[0037] (3) transforming the recombinant plasmid pET-28a(+)-ULP obtained in step (2) into Escherichia coli BL21(DE3);
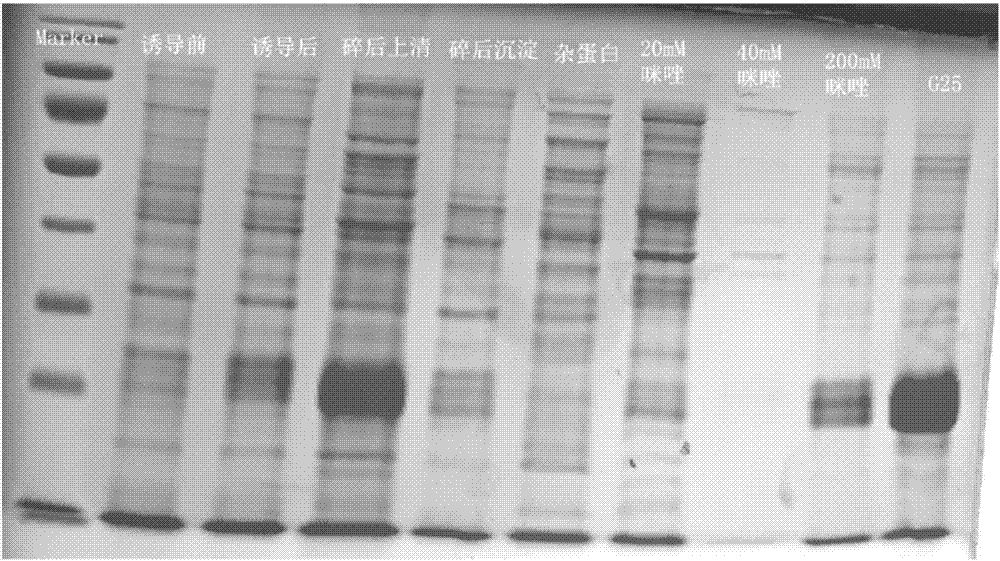
[0038](4) Cultivate the genetically engineered bacteria obtained in step (3): Add 5ul of the frozen strain to 20ml ordinary culture medium containing 10ul 100mg / ml kanamycin, 37°C, 200rpm, and cultivate for 14h (overnight); 50ul 100mg / ml Add ml kanamycin to 200ml ord...
Embodiment 2
[0060] Application of Example 2 Truncated GDF11 in Stimulating Fibroblast Collagen Secretion
[0061] Using the picrosirius red ELISA detection kit, the mouse suckling mouse fibroblasts were washed and blotted dry with PBS, and then divided into 4 groups, and purified truncated GDF11 (implemented with 10ng / ml, 50ng / ml, 500ng / ml respectively) prepared in Example 1) to stimulate the cells for 48 hours respectively, and the group without truncated GDF11 was set as the control. After 48 hours, lyse with collagen lysate on ice for 30 min, sonicate for 5 min x 5 times, centrifuge at low temperature (13,500 rpm to lyse cells), take the supernatant in another EP tube, and use an enzyme-linked immunosorbent assay at a wavelength of 562 nm The absorbance of the protein was measured, and the protein concentration was converted according to the standard curve. Place the remaining supernatant in a light-proof tube, stain with the dye in the kit, and stain in the dark at 4°C for 30 minutes...
Embodiment 3
[0063] Example 3 Application of truncated GDF11 in promoting the expression and phosphorylation of Smad2 / 3
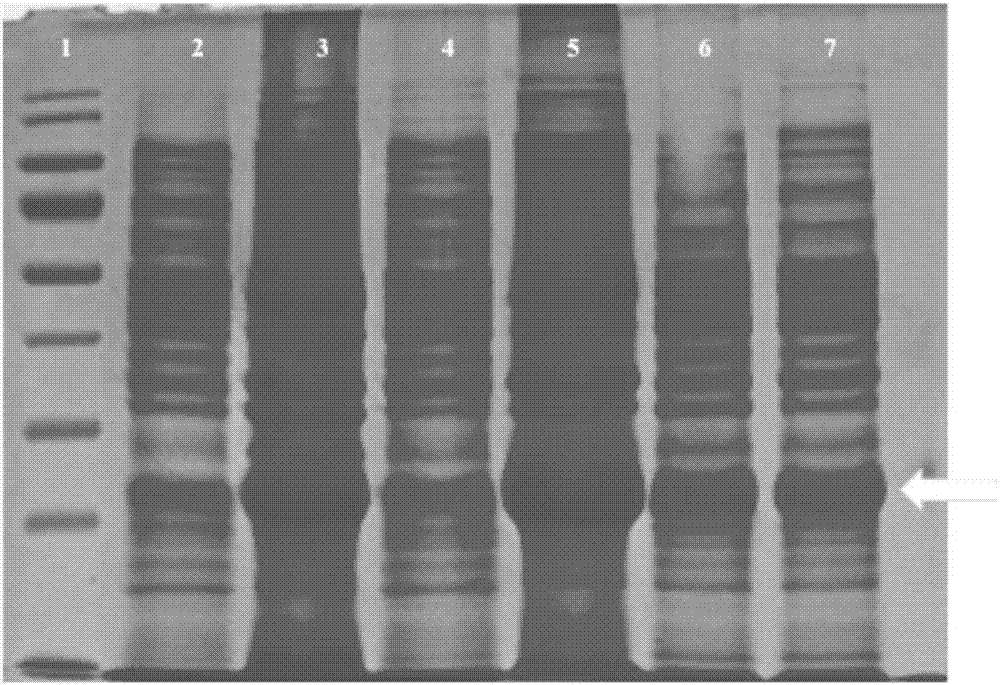
[0064] Primary culture neonatal rat cardiac fibroblasts in a six-well plate, add purified truncated GDF11 (prepared in Example 1) or full-length GDF11 to treat for 25 minutes and 1 hour respectively, then wash twice with PBS, add 50ul of lysate and 5ul of Protease inhibitors, 2ulPMSF, scraped off the cells, transferred to a centrifuge tube, placed on ice for 30min, then centrifuged at 12000g for 50min, collected the supernatant to obtain the total protein, measured the protein concentration with BCA, and detected the truncated GDF11 and full-length GDF11 by western blotting Effects on the expression and phosphorylation level of Smad2 / 3 in cardiac fibroblasts.
[0065] The result is as Figure 7 shown, from Figure 7 It can be seen from the results that the truncated GDF11 of the present invention can promote the phosphorylation of Smad2 / 3, and has a stronger effect th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com