Benzisothiazolone derivative and preparation method and application thereof
A technology of benzisothiazolone and derivatives, applied to benzisothiazolone derivatives, preparation of benzisothiazolone derivatives, application fields of benzisothiazolone derivatives in antifungal and antibacterial , can solve the problems of VOC, easy to lose, easy to be absorbed by the human body, etc., and achieve the effect of not easy to absorb, not easy to lose, and excellent mildew resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
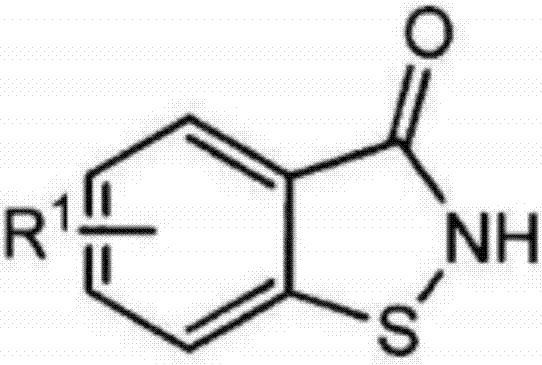
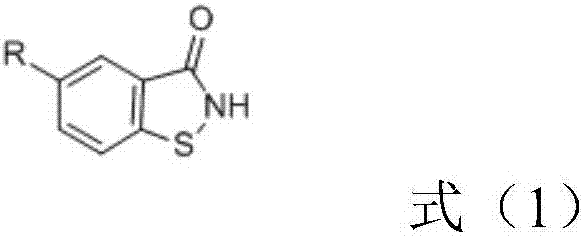
[0048] In the presence of 450g of DMF and 107g of sodium carbonate, 185.5g of 5-chloro-1,2-benzisothiazol-3-ketone and 80g of brominated paraffin (halogen atom content is 40-44% by weight, purchased from Wuyi Diwang Chemical Auxiliary Co., Ltd.) for reflux reaction for 10 hours, washed with water after the reaction (the unreacted raw material 5-chloro-1,2-benzisothiazol-3-one was washed away), and the crude product , decolorized to obtain benzisothiazolone derivative M1, the yield (based on 5-chloro-1,2-benzisothiazol-3-one, the same below) was 92%.
Embodiment 2
[0050] In the presence of 400g of DMF and 80g of lithium carbonate, 165g of 5-methyl-1,2-benzisothiazol-3-ketone and 90g of chlorinated paraffin (halogen atom content is 50-54% by weight, , purchased from Yangzhou Keli Chemical Co., Ltd.) for reflux reaction for 15h, washed with water after the reaction (the unreacted raw material 5-methyl-1,2-benzisothiazol-3-one was washed away), and the crude product was obtained. After decolorization, the benzisothiazolone derivative M2 was obtained with a yield of 93%.
Embodiment 3
[0052] In the presence of 500g of acetonitrile and 139g of potassium carbonate, 151g of 1,2-benzisothiazol-3-one and 80g of chlorinated paraffin (halogen atom content is 68-72% by weight, purchased from Yangzhou Keli Chemical Co., Ltd.) for reflux reaction for 20h, after the reaction was completed, washed with water (the unreacted raw material 1,2-benzisothiazol-3-one was washed away), obtained the crude product, decolorized, and obtained benzisothiazolone derivatives M3, 92% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com