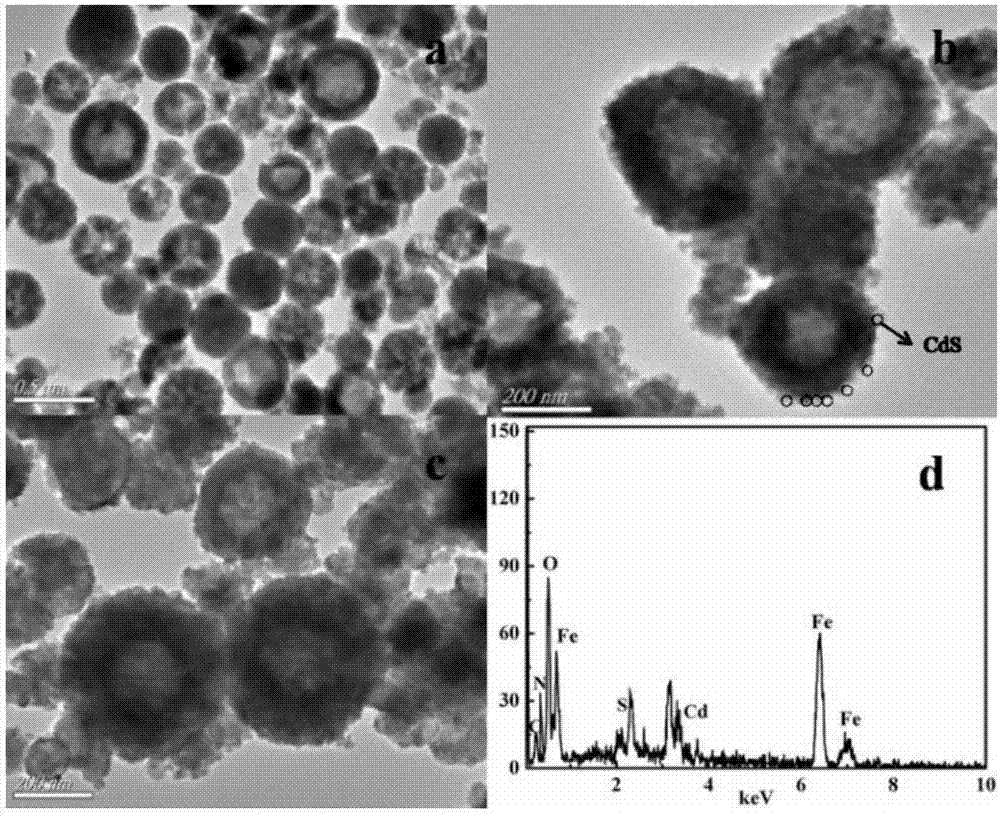
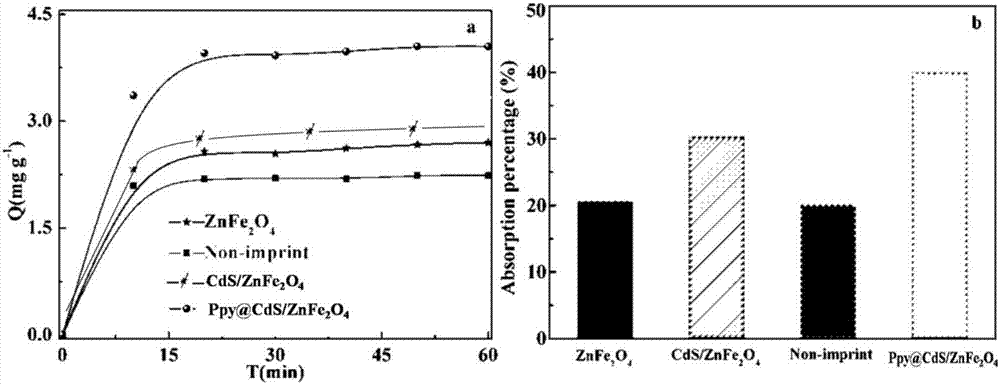
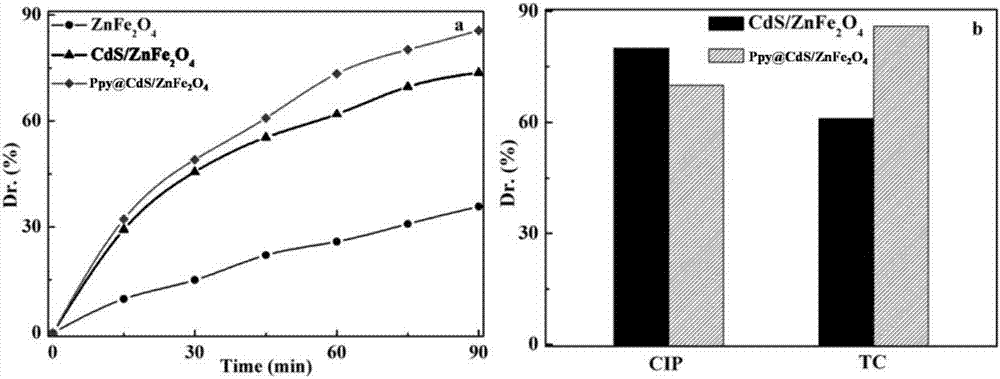
Magnetic composite photocatalyst Ppy@CdS/ZnFe2O4 and preparation method and application thereof
A znfe2o4, composite light technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of inability to selectively degrade single and other targets, long degradation time and other problems, to achieve the effects of improving separation efficiency and light absorption utilization, improving catalytic activity, and inhibiting photocorrosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Step 1. ZnFe 2 o 4 Preparation of: FeCl 3 ·6H 2 O was added to ethylene glycol and stirred until FeCl 3 ·6H 2 O is completely dissolved, add anhydrous sodium acetate, then add ZnCl 2 Continue to stir until the solution becomes clear, then transfer the obtained mixed solution A to a high-pressure reactor and put it in an oven for solvothermal reaction for a period of time; after the oven is naturally cooled to room temperature, take out the reactor and wash the inside of the reactor. The solid sample, which is then dried for later use, is denoted as ZnFe 2 o 4 ;
[0029] Step 2. CdS / ZnFe 2 o 4 Preparation: ZnFe 2 o 4 Disperse in deionized water, then add cadmium sulfate and sulfur urine, stir until uniformly dispersed, then react the obtained dispersion B in a constant temperature water bath and inert gas protection conditions, and wash the solid after the reaction is completed and naturally cooled to room temperature The product, dried for future use, is rec...
Embodiment 1
[0032] In step 1, the FeCl 3 ·6H 2 O and ZnCl 2 The consumption is 2mmol and 1mmol, the consumption of anhydrous sodium acetate is 20mmol, the consumption of ethylene glycol is 40mL, the hydrothermal temperature in the stainless steel autoclave is 210 ℃, and described time is 24h.
[0033] In step 2, the ZnFe 2 o 4 , cadmium sulfate, thiourea, deionized water, and ammonia water are used in the order of 0.1g, 10mL, 0.05g, 0.03g, 50mL, and 5mL.
[0034] In step 3, the CdS / ZnFe 2 o 4 , chloroform, pyrrole, TRIM, AIBN, and the consumption of tetracycline are successively 0.1g, 10mL, 0.2mL, 0.05mL, 0.002mL, 0.002g; the microwave reaction bottle is put into a microwave reactor, and the setting condition is 40 ℃, 600W power, 800 rotation speed, react for 1h, and finally get imprinted Ppy@CdS / ZnFe 2 o 4 catalyst of light.
Embodiment 2
[0036] In step 1, the FeCl 3 ·6H 2 O and ZnCl 2 The consumption of sodium acetate is 4mmol and 2mmol, the consumption of anhydrous sodium acetate is 40mmol, the consumption of ethylene glycol is 100mL, and the hydrothermal temperature in stainless steel autoclave is 210 ℃, and described time is 72h.
[0037] In step 2, the ZnFe 2 o 4 , cadmium sulfate, thiourea, deionized water, and ammonia water are used in the order of 0.4g, 30mL, 0.15g, 0.09g, 150mL, and 15mL.
[0038] In step 3, the CdS / ZnFe 2 o 4 , chloroform, pyrrole, TRIM, AIBN, and the consumption of tetracycline are 0.3g, 20mL, 0.6mL, 0.15mL, 0.08mL, 0.08g; ℃, 600W power, 800 rotation speed, react for 1h, and finally get imprinted Ppy@CdS / ZnFe 2 o 4 catalyst of light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com