Preparing method of high-purity fluoroethylene carbonate
The technology of pure fluoroethylene carbonate and chloroethylene carbonate is applied in the field of preparation of high-purity fluoroethylene carbonate, which can solve the problems of high equipment requirements and low product purity, and achieves the reduction of impurity content, high purity, Process Control Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
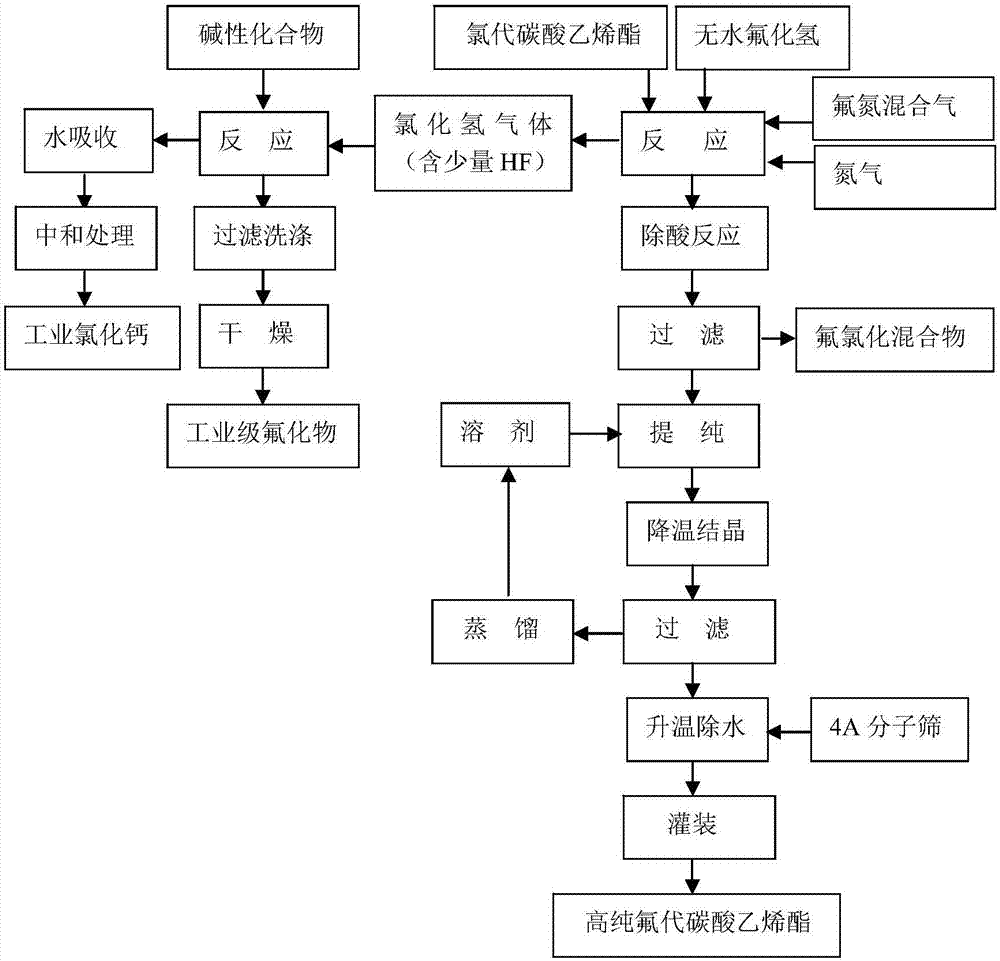
[0030] The preparation method of the high-purity fluoroethylene carbonate of the present embodiment, technological process is as follows figure 1 shown, including the following steps:
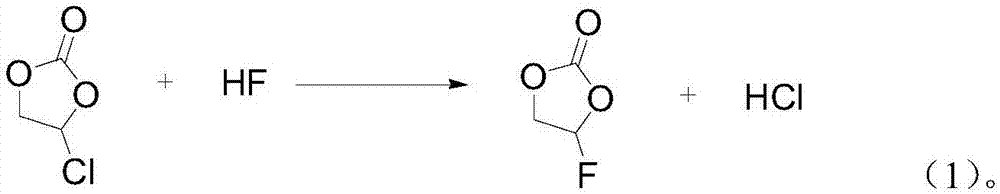
[0031] 1) 1 kg of chloroethylene carbonate raw material and anhydrous hydrogen fluoride liquid are passed into the microchannel reactor at the same time in a molar ratio of 1:1 for reaction, the reaction temperature is -15°C, and the reaction time is 7 minutes;
[0032] 2) Step 1) After the reaction is complete, transfer the reaction liquid and reaction gas to the tank reactor, and pass dry high-purity nitrogen gas to replace the hydrogen chloride gas in the tank reactor. The temperature in the reactor is controlled at -15°C, and the nitrogen gas The time is 3min;
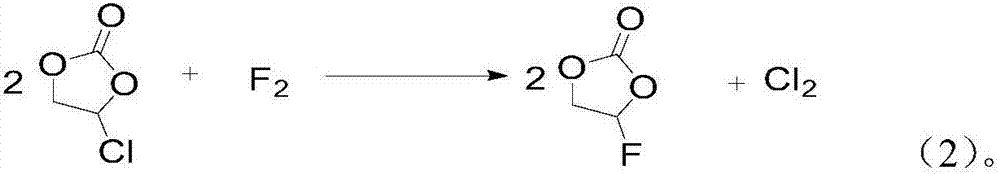
[0033] 3) Step 2) After the replacement is complete, feed a fluorine-nitrogen mixed gas with a fluorine-containing gas volume content of 5% and react with unreacted chloroethylene carbonate. In the fluorine-nitrogen mixed gas, fluo...
Embodiment 2
[0041] The preparation method of the high-purity fluoroethylene carbonate of the present embodiment may further comprise the steps:
[0042] 1) Feed 1 kg of chloroethylene carbonate raw material and anhydrous hydrogen fluoride liquid into a microchannel crystallizer at the same time in a molar ratio of 1:1.1 for reaction, the reaction temperature is 0°C, and the reaction time is 10 minutes;
[0043] 2) Step 1) After the reaction is complete, transfer the reaction liquid and reaction gas to the tank reactor, and pass dry high-purity nitrogen gas to replace the hydrogen chloride gas in the tank reactor. The temperature in the reactor is controlled at 0°C, and the nitrogen gas The time is 10 minutes;
[0044] 3) Step 2) After the replacement is complete, feed a fluorine-nitrogen mixed gas with a fluorine-containing gas volume content of 5% and react with unreacted chloroethylene carbonate. In the fluorine-nitrogen mixed gas, fluorine and chloroethylene carbonate The molar ratio ...
Embodiment 3
[0052] The preparation method of the high-purity fluoroethylene carbonate of the present embodiment may further comprise the steps:
[0053] 1) Feed 1 kg of chloroethylene carbonate raw material and anhydrous hydrogen fluoride liquid into a microchannel crystallizer at the same time in a molar ratio of 1:1.15 for reaction, the reaction temperature is 16°C, and the reaction time is 25 minutes;
[0054] 2) Step 1) After the reaction is complete, transfer the reaction liquid and reaction gas to the tank reactor, and pass dry high-purity nitrogen gas to replace the hydrogen chloride gas in the tank reactor. The temperature in the reactor is controlled at 18°C, and the nitrogen gas The time is 10 minutes;
[0055] 3) Step 2) After the replacement is complete, feed a fluorine-nitrogen mixed gas with a fluorine-containing gas volume content of 10% and react with unreacted chloroethylene carbonate. In the fluorine-nitrogen mixed gas, fluorine and chloroethylene carbonate The molar ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com