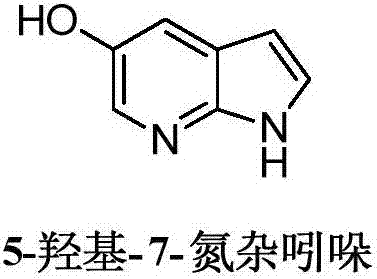
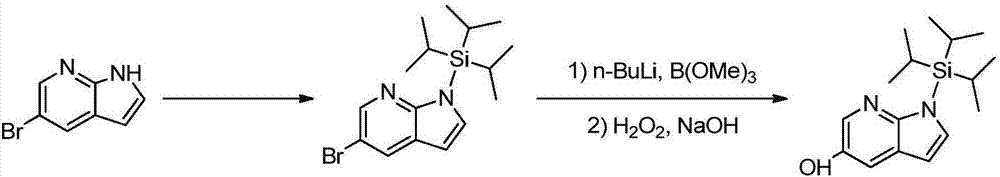
Method for synthesizing Venetoclax key intermediates
A synthesis method and intermediate technology, applied in the field of drug synthesis, can solve the problems of not obtaining 5-hydroxy-7-azaind, etc., and achieve the effect of avoiding column chromatography purification operation, avoiding production conditions, and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of 7-azaindole-5-pinacol borate: add 11g 5-bromo-7-azaindole, 20g double pinacol borate, 15g potassium acetate into a three-necked flask , 110mL dioxane, nitrogen replacement, add 30mg Pd(dppf)Cl 2 , The temperature was raised to 100°C and reacted for 24h (HPLC monitoring of 5-bromo-7-azaindole reaction was complete). The reaction solution was concentrated to dryness, 220mL ethyl acetate and 220mL water were added and stirred, the organic phase was separated, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain a theoretical product, which was directly put into the next step reaction.
[0032] (2) Synthesis of 5-hydroxy-7-azaindole: Add the above 7-azaindole-5-pinacol borate, 220mL tetrahydrofuran, 220mL 2M sodium hydroxide solution and 17.5g tetrahydrofuran to the reaction flask. Sodium perborate, react at room temperature for 2h (TLC monitors that the reaction of 7-azaindole-5-pinacol borate is complete), separate the organic phase, ad...
Embodiment 2
[0034] (1) Synthesis of 7-azaindole-5-pinacol borate: add 11g 5-bromo-7-azaindole, 20g double pinacol borate, 15g potassium acetate into a three-necked flask , 110mL dioxane, nitrogen replacement, add 30mg Pd (PPh 3 ) 4 , The temperature was raised to 100°C and reacted for 24h (HPLC monitors that the reaction of 5-bromo-7-azaindole is complete, and the bromine product 7-azaindole accounts for about 4.3%). The reaction solution was concentrated to dryness, 220mL ethyl acetate and 220mL water were added to stir, the organic phase was separated, dried over anhydrous sodium sulfate, concentrated to dryness, and put into the next step reaction directly according to the theoretical amount of product.
[0035] (2) Synthesis of 5-hydroxy-7-azaindole: Add the above 7-azaindole-5-pinacol borate, 220mL tetrahydrofuran, 220mL 2M sodium hydroxide solution and 17.5g tetrahydrofuran to the reaction flask. Sodium perborate, react at room temperature for 2h (TLC monitors that the reaction of 7-az...
Embodiment 3
[0037] (1) Synthesis of 7-azaindole-5-pinacol borate: add 11g 5-bromo-7-azaindole, 20g double pinacol borate, 15g potassium acetate into a three-necked flask , 110mL dioxane, nitrogen replacement, add 30mg Pd (PPh 3 ) 2 Cl 2 , The temperature was raised to 100°C and reacted for 24h (HPLC monitors that the reaction of 5-bromo-7-azaindole is complete, and the bromine product 7-azaindole accounts for about 6.5%). The reaction solution was concentrated to dryness, 220mL ethyl acetate and 220mL water were added and stirred, the organic phase was separated, dried over anhydrous sodium sulfate, concentrated to dryness, and put into the next step reaction directly according to the theoretical amount of product.
[0038] (2) Synthesis of 5-hydroxy-7-azaindole: add the above 7-azaindole-5-pinacol borate, 220mL tetrahydrofuran, 220mL 2M sodium hydroxide solution and 17.5g tetrahydrofuran to the reaction flask. Sodium perborate, react at room temperature for 2h (TLC monitors 7-azaindole-5-pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com