Preparation method of reductive-response amphipathic wormlike monomolecular prodrug
An amphiphilic, worm-like technology, used in pharmaceutical formulations, organic active ingredients, and non-active ingredients medical preparations, etc., can solve the problems of uncontrollable release, low drug loading rate, poor selectivity, etc., and achieve enhanced internalization. effect, enhanced upload capacity and selective release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
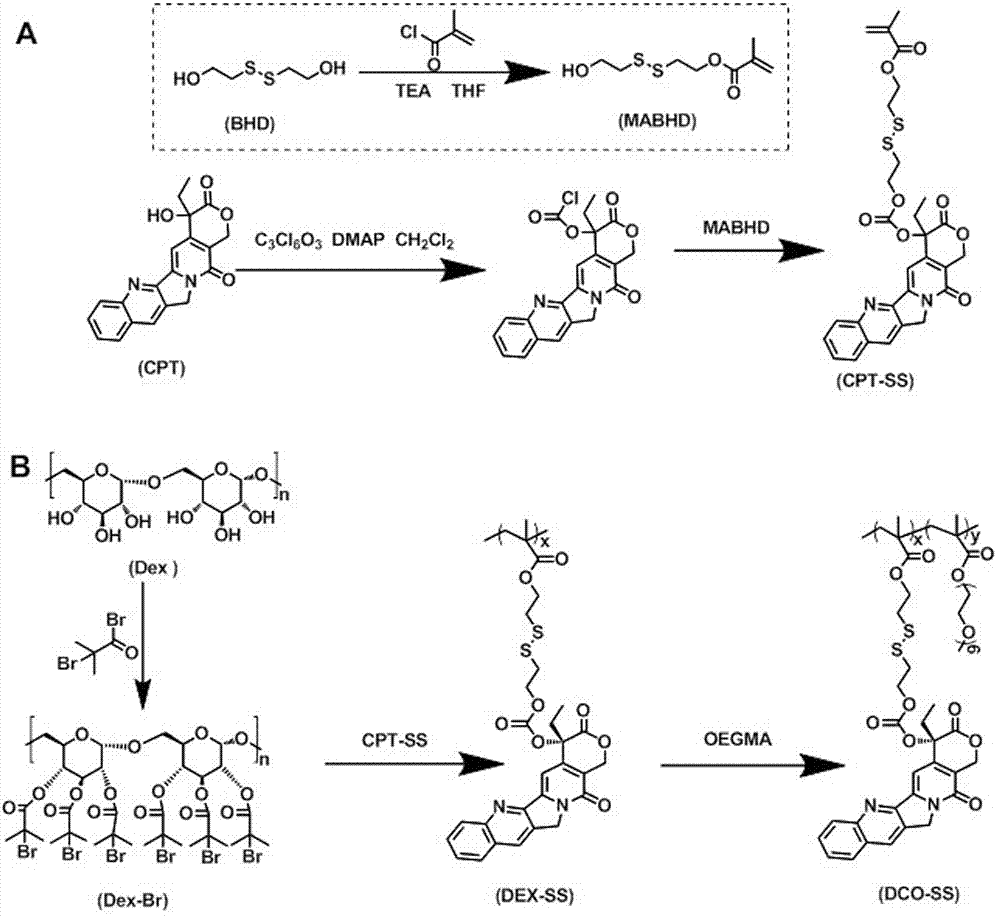
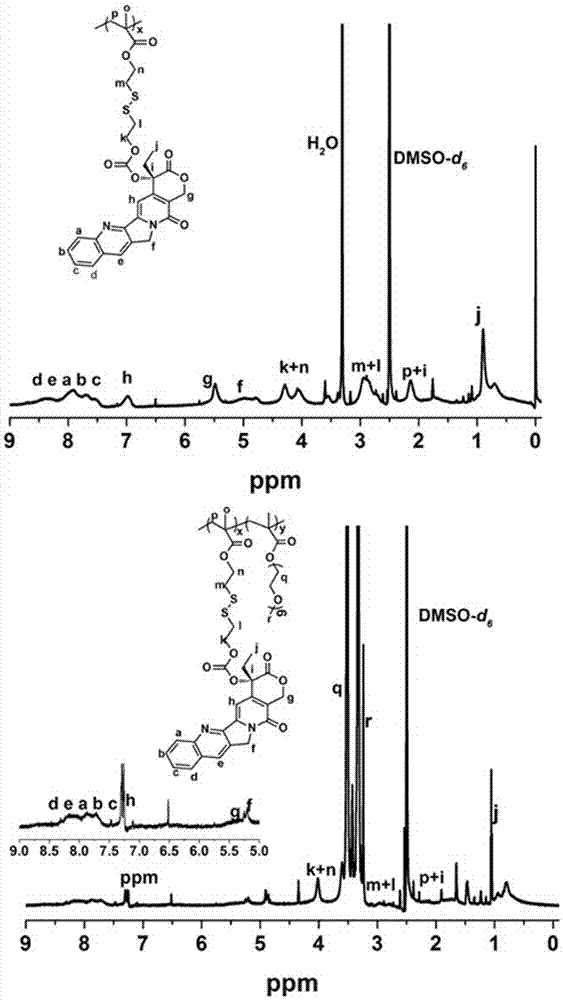
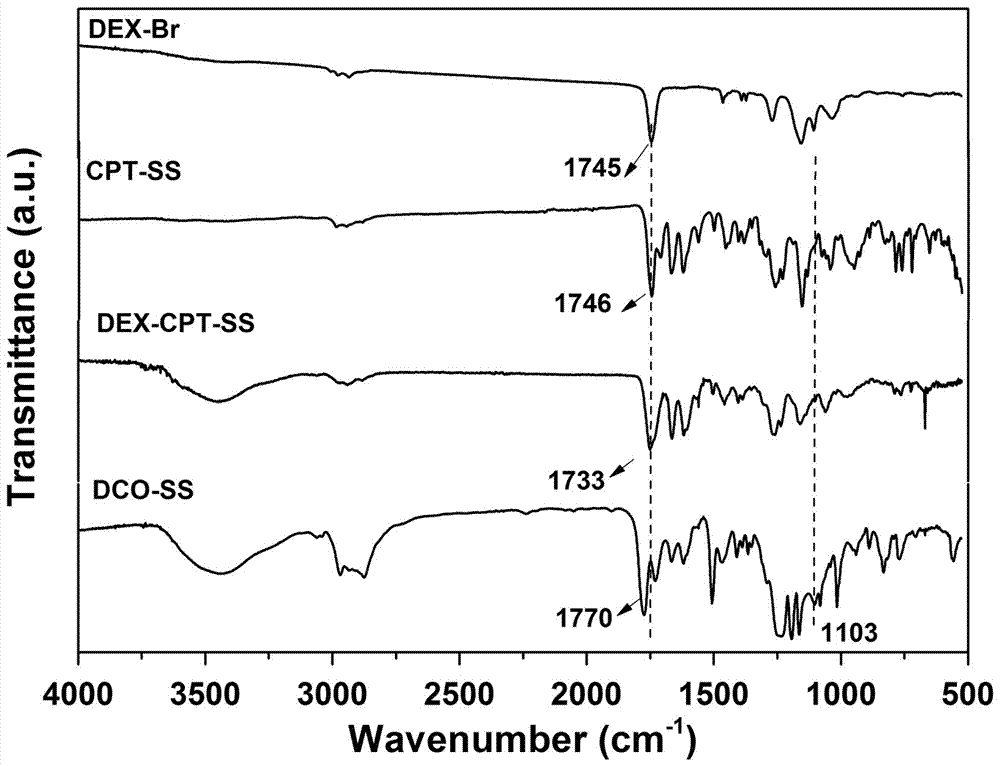
[0027] Example 1 Preparation of reductively responsive amphiphilic worm-like single-molecule prodrug DCO-SS
[0028] (1) Preparation of CPT precursor CPT-SS with reductive response: Under argon (Ar) atmosphere, ① 2-hydroxyethyl disulfide (BHD, 6.25g, 40.5mmol), methacryloyl chloride ( 2.2mL, 22.73mmoL, dissolved in 15 mL of anhydrous THF, slowly added dropwise to the system), tetrahydrofuran (THF, 50mL) and triethylamine (TEA, 3.3mL, 24mmoL) were mixed and stirred overnight, column purified to obtain MABHD; ② CPT (1.39g, 4mmoL), triphosgene (BTC, 500mg, 1.685mmoL, dissolved in 10mL anhydrous DCM, slowly added dropwise to the system), 4-dimethylaminopyridine (DMAP, 1.56g, 11.3mmoL ) mixture was dispersed into 60mL of anhydrous DCM, reacted for 30min, then added dropwise MABHD (976mg, 2.375mmoL, dissolved in 10 mL of anhydrous THF, slowly added dropwise to the system), stirred overnight, and purified to obtain the precursor CPT- SS;
[0029](2) Preparation of the drug initiato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com