Method for detecting genotoxic impurities in AL58805 bulk drug or medicinal preparation by using high-performance liquid chromatography
An AL58805, high-performance liquid chromatography technology, applied in the field of analysis of genotoxic impurities, can solve the problems of high price, restricted promotion and use, etc., and achieve the effects of strong specificity, high sensitivity and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
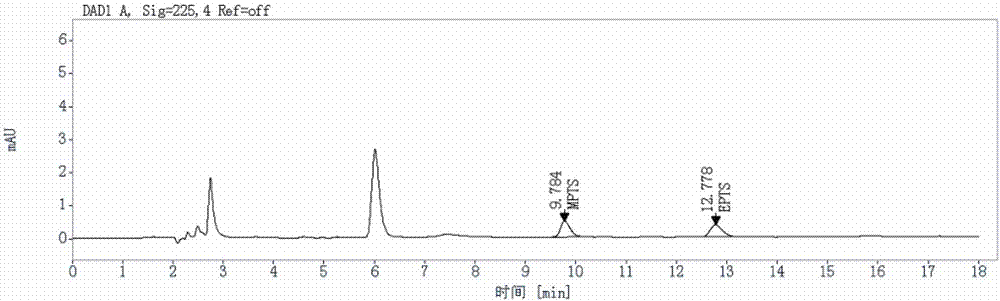
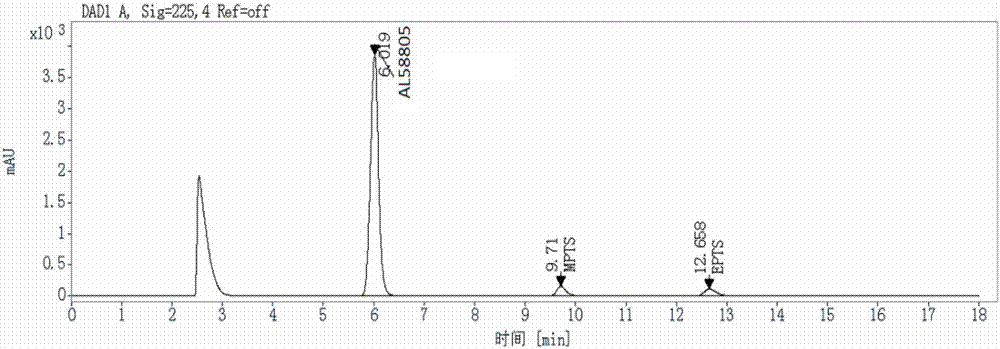
[0041] 1. Preparation of standard stock solution
[0042] Accurately weigh an appropriate amount of methyl p-toluenesulfonate and ethyl p-toluenesulfonate reference substance respectively, add acetonitrile to dissolve and dilute to make a solution containing about 1mg in each 1mL as a standard stock solution, and then use acetonitrile-water (50:50V / V) gradually diluted to the desired concentration.
[0043] 2. Liquid Chromatography Conditions
[0044] The chromatographic column is InertSustain C18 (4.6×250mm, 5μm); the mobile phase is 15mmol / L phosphoric acid aqueous solution-acetonitrile
[0045] (50:50V / V); the detection wavelength is 225nm; the flow rate is 1mL / min; the column temperature is 35°C; the injection volume is 20μL; the elution time is 18min.
[0046] 3. Sample pretreatment method
[0047] Take about 100mg of the AL58805 test sample, put it in a 10mL measuring bottle, add an appropriate amount of acetonitrile-water (50:50V / V) to dissolve, adjust the pH to 9 w...
Embodiment 2
[0077] 1, the preparation of standard stock solution is identical with embodiment 1;
[0078] 2. In liquid chromatography conditions: the mobile phase is 5mmol / L phosphoric acid aqueous solution-acetonitrile (50:50V / V); the rest are the same as in Example 1.
[0079] 3. Sample pretreatment: the same as in Example 1.
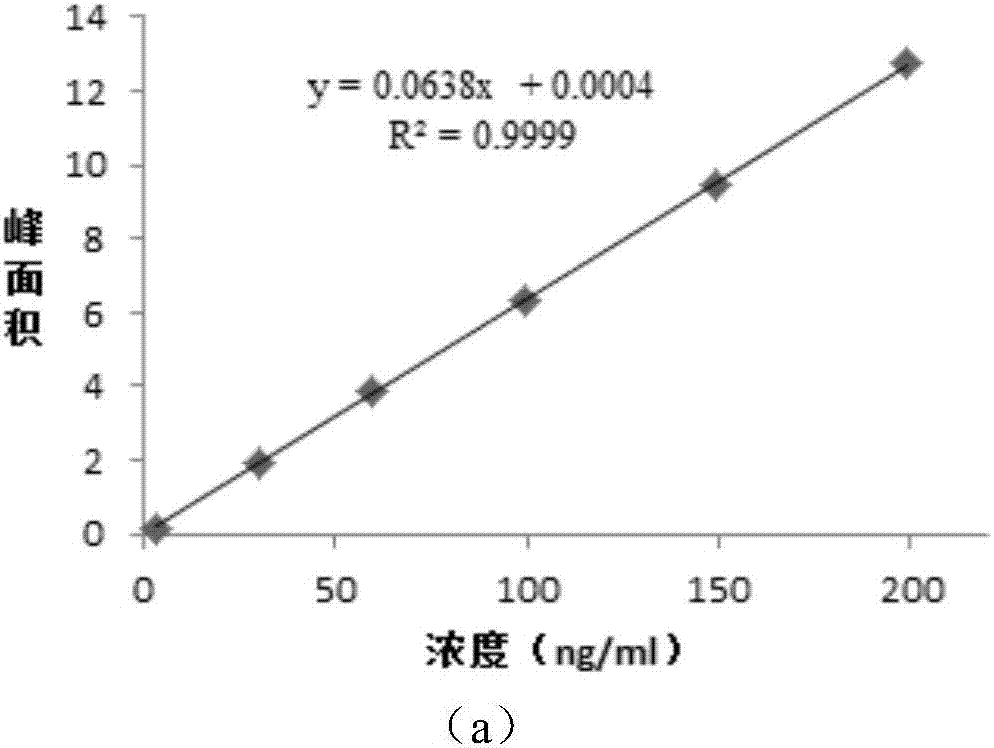
[0080] 4, methodological verification is identical with embodiment 1, and the result shows, the average rate of recovery of methyl p-toluenesulfonate (the standard in Pharmacopoeia is 80-115%) is 89.0%, and RSD is 1.6%, and the average recovery rate of ethyl p-toluenesulfonate Rate (standard is 80-115% in Pharmacopoeia) is 101.7%, and RSD is 2.3%, and the accuracy that this method detects methyl p-toluenesulfonate, ethyl p-toluenesulfonate is good.
Embodiment 3
[0082] 1, the preparation of standard stock solution is identical with embodiment 1;
[0083] 2. The mobile phase in the liquid chromatography conditions is 50mmol / L phosphoric acid aqueous solution-acetonitrile (50:50V / V); the rest are the same as in Example 1.
[0084] 3. Sample pretreatment: the same as in Example 1.
[0085] 4, methodological verification is identical with embodiment 1, and the result shows, the average recovery rate of methyl p-toluenesulfonate is 93.1%, and RSD is 1.7%, and the average recovery rate of ethyl p-toluenesulfonate is 102.1%, and RSD is 2.7%, The method has good accuracy in detecting methyl p-toluenesulfonate and ethyl p-toluenesulfonate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com