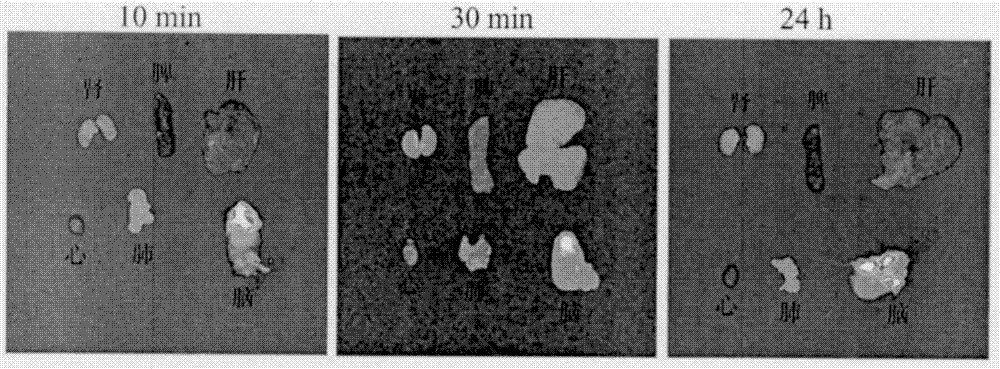
Functional curcumin nano-drug for diagnosis and treatment of cerebral diseases and application of nano-drug
A technology of nano-drugs and curcumin derivatives, which is applied in drug combination, drug delivery, nervous system diseases, etc., can solve the problems of poor water solubility and chemical instability of curcumin, and achieve improved water solubility and stability, Increased pharmacological activity, increased permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
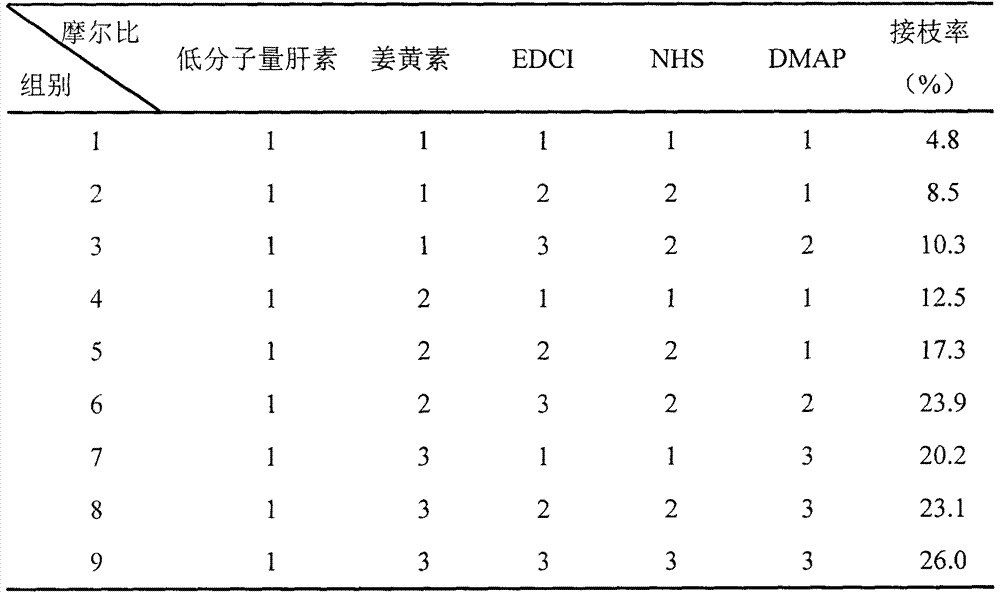
[0034] Embodiment 1: ester bond is the synthesis and grafting rate of the curcumin derivative of linking arm
[0035] Curcumin and low-molecular-weight heparin were dissolved in appropriate solvents as 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI), N-hydroxysuccinimide (NHS) , 4-dimethylaminopyridine (DMAP) as an activator. Dissolve low-molecular-weight heparin in formamide in an oil bath at 40°C for about 1 hour, then add EDCI under ice-cooling, add NHS after activation for 30 minutes, activate in ice-bathing for 2 hours, then add curcumin and DMAP, protected from light, activated in an ice bath for 30 minutes, then moved to room temperature and protected from light for 24 hours. The above process was protected with nitrogen. After the reaction was completed, excess ice acetone (3-5 times the amount) was added to precipitate the product, and the precipitate was obtained by suction filtration. The resulting precipitate was redissolved in water, sonicated b...
Embodiment 2
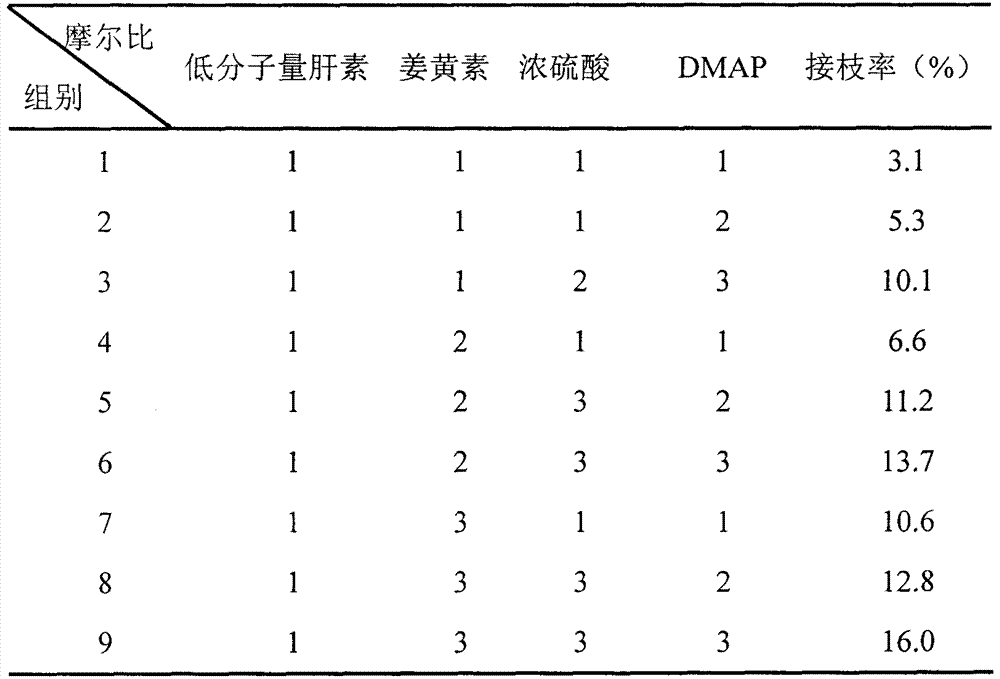
[0038] Embodiment 2: ether bond is the synthesis and grafting rate of the curcumin derivative of linking arm
[0039] Low-molecular-weight heparin and curcumin were placed in round-bottomed flasks respectively, N,N-dimethylformamide was added, stirred to make it dissolve, alkalizing reagent 4-dimethylaminopyridine (DMAP) was added in the curcumin solution, To achieve the purpose of activating the phenolic hydroxyl group; slowly add the activated curcumin to the low molecular weight heparin solution at an interval of 5 seconds per drop, and at the same time add concentrated sulfuric acid as a water-absorbing agent, and stir at room temperature for 24 hours. After the reaction is completed, add ice acetone to precipitate the product, pump Filter the precipitate; add water to redissolve the precipitate, dialyze in water for 1 day, and freeze-dry to obtain the final product - curcumin derivative. The grafting ratio of the obtained product is shown in Table 2.
[0040] Table 2 eth...
Embodiment 3
[0042] Embodiment 3: Ethylenediamine is the synthesis of the curcumin derivative of connecting arm
[0043] Take 2 mol of low molecular weight heparin and place it in a round bottom flask, add 50 mL of formamide to dissolve it, add 8 mol of ethylenediamine, stir for 2 min with magnetic force, then add 3 mol of 1-ethyl-(3-dimethylaminopropyl) carbodiimide ( EDC) and 4.5mol hydroxysuccinimide (NHS), reacted at room temperature for 12 hours, added acetone to precipitate the product after the reaction, filtered to obtain the precipitate, redissolved in water, dialyzed for 2 days, and freeze-dried to obtain a heparin active intermediate with a free one-terminal amino group . Dissolve the heparin active intermediate obtained above in a methanol-water mixed solution, avoid light, and slowly add 6 mol of curcumin in methanol solution dropwise under magnetic stirring at 50°C for 1.5 hours. Take the product by rotary evaporator to remove methanol, add a small amount of water to mix, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com