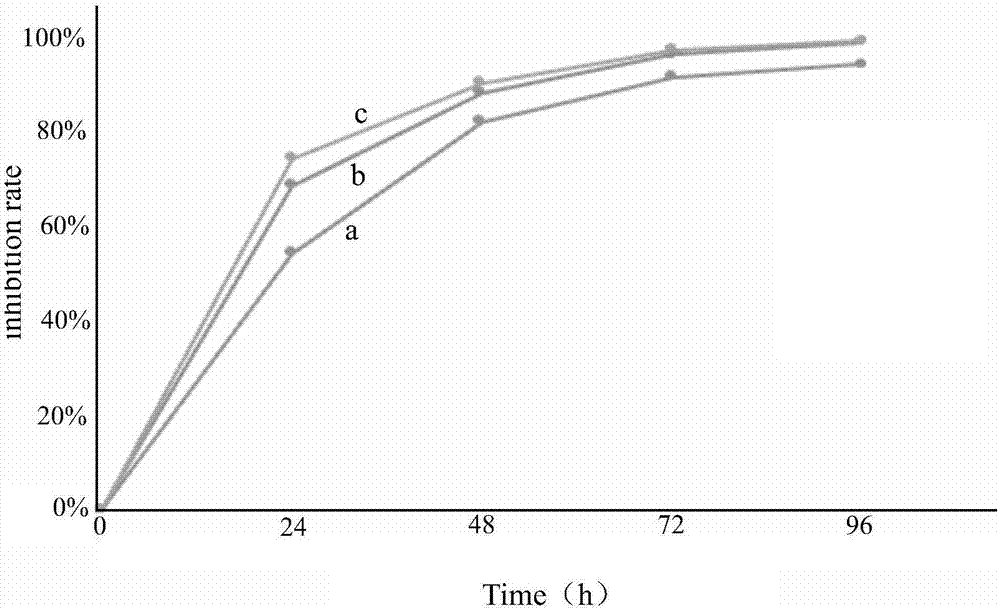
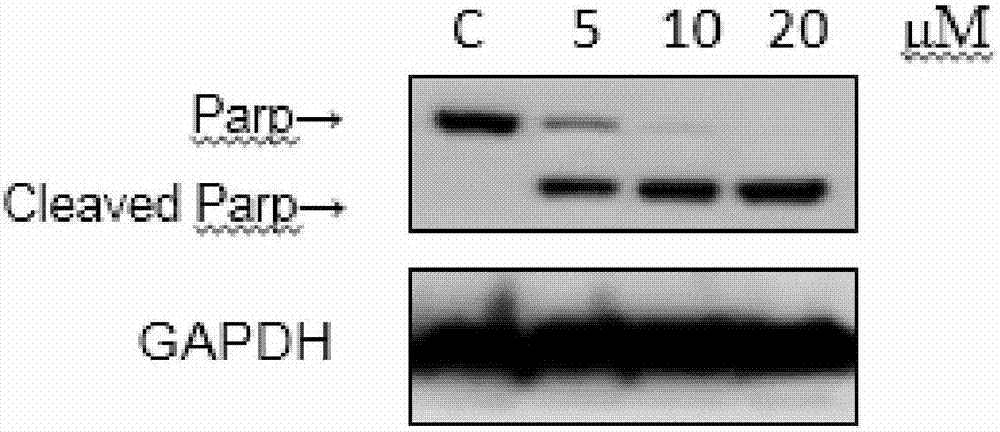
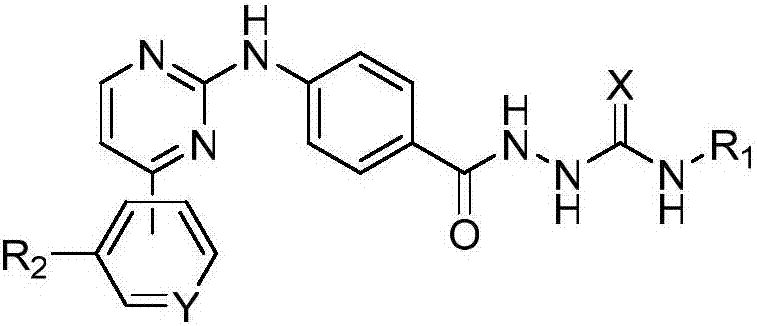
4-((4-substituted aryl-2-pyrimidinyl) amino) benzoyl hydrazide derivative as well as preparation method and application thereof
A technology for benzoyl hydrazide and derivatives, which is applied in the field of 4-amino) benzoyl hydrazide derivatives and their preparation, can solve the problems of large toxic and side effects, few tumor drugs, low bioavailability and the like, and achieves a simple reaction process. Easy to control, low cost of synthetic reaction, inhibition of inducing tumor cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of ethyl p-guanidine benzoate nitrate
[0027] In a 200mL dry single-neck bottle, first add ethyl p-aminobenzoate (5.0g, 0.030mol), cyanamide (50%w / w, 7.6g, 0.091mol), absolute ethanol (20mL); Concentrated hydrochloric acid (3.8 mL, 0.045 mol) was added dropwise under stirring, and the temperature was raised to reflux after the dropwise addition. After 24 hours of reaction, TLC detected that the reaction was complete, and the reaction was stopped. The reaction solution was concentrated under reduced pressure to remove the solvent, then 50 mL of water was added, and then an aqueous solution of ammonium nitrate (4.8 g, 0.061 mol) was added dropwise at 0°C. After the addition was completed, the reaction was incubated for 1 hour, filtered, the filter cake was collected, and dried to obtain a white solid The product was 6.3 g of ethyl guanidine benzoate nitrate, and the yield was 78%.
Embodiment 2
[0028] Example 2: Synthesis of 1-(2-pyridyl)-3-(dimethylamino)-2-propene-1-one
[0029] Add 2-acetylpyridine (5.0g, 0.041mol) and N,N-dimethylformamide dimethyl acetal (5.4g, 0.045mol) sequentially into a dry 100mL single-necked bottle, heat to 110°C, and reflux After 18 hours of reaction, TLC detected that the reaction was complete, and the reaction was stopped. After the reaction solution was concentrated under reduced pressure, 5 mL of ethyl acetate was added, stirred at room temperature for 1 h, filtered with suction, the filter cake was collected, and dried to obtain 4.2 g of a yellow-green solid with a yield of 58%. Spectral data: 1 H NMR (600MHz, DMSO-d 6 ):8.55~8.69(m,1H),7.98(d,J=7.7Hz,1H),7.91(dt,J=1.7,7.66Hz,1H),7.80(d,J=12.6Hz,1H),7.50 (ddd,J=1.2,4.77,7.52Hz,1H),6.38(d,J=10.6Hz,1H),3.18(s,3H),2.92(s,3H).
Embodiment 3
[0030] Embodiment 3: Synthesis of ethyl 4-((4-(2-pyridyl)-2-pyrimidinyl) amino)benzoate
[0031]Add 1-(2-pyridyl)-3-(dimethylamino)-2-propen-1-one (1.00 g, 0.0056 mol) and ethyl p-guanidinobenzoate nitric acid to a 50 mL dry single-necked bottle successively Salt (1.53g, 0.0056mol), sodium hydroxide solid (0.273g, 0.0068mol), absolute ethanol (10mL), heated to reflux, reacted for 18h, TLC detected that the reaction was complete, stop the reaction. After cooling to room temperature, it was filtered with suction, and the filter cake was collected and dried to obtain 1.52 g of off-white solid with a yield of 84%. Spectral data: 1 HNMR (600MHz, CDCl 3 ):8.73(d,J=4.4Hz,1H),8.62(d,J=4.9Hz,1H),8.42(d,J=7.7Hz,1H),8.07(d,J=8.2Hz,2H), 7.89-7.91(m,1H),7.87(d,J=5.6Hz,1H),7.81(d,J=8.0Hz,2H),7.56(br.s.,1H), 7.40~7.45(m,1H ), 4.38(q, J=7.0Hz, 2H), 1.41(t, J=6.9Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com