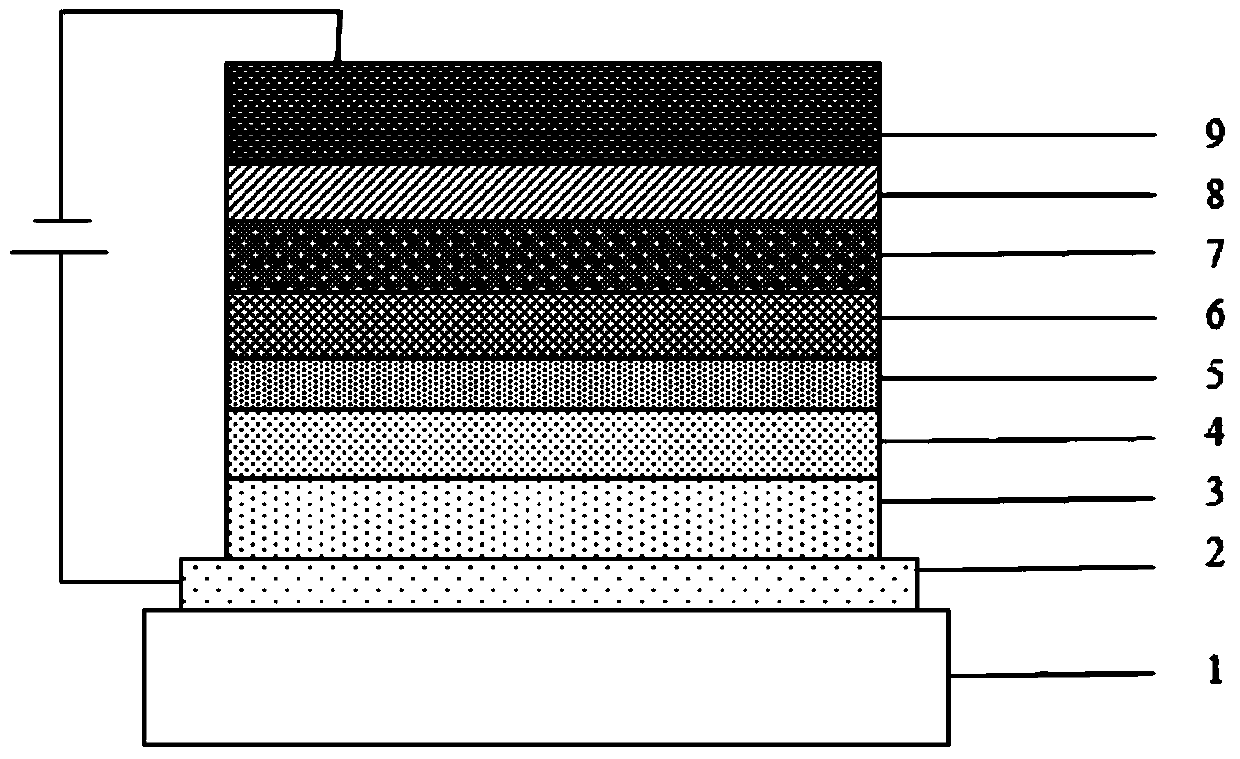
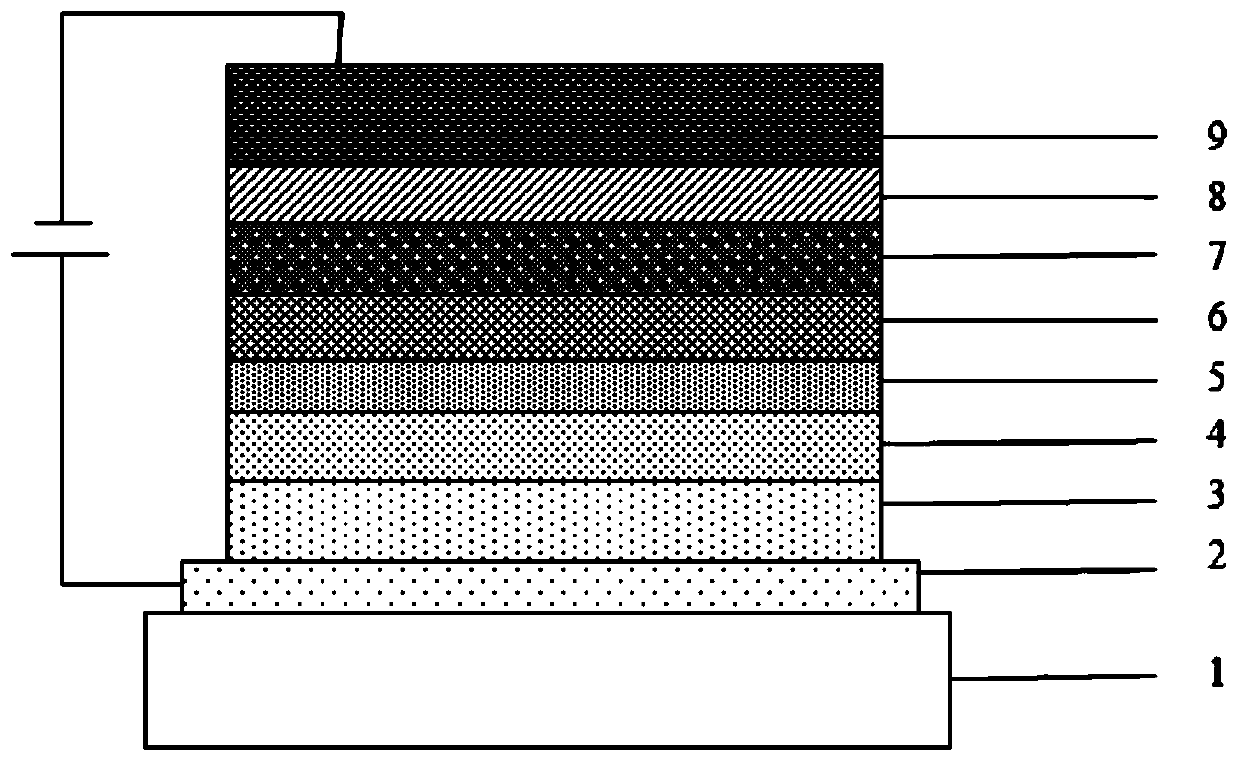
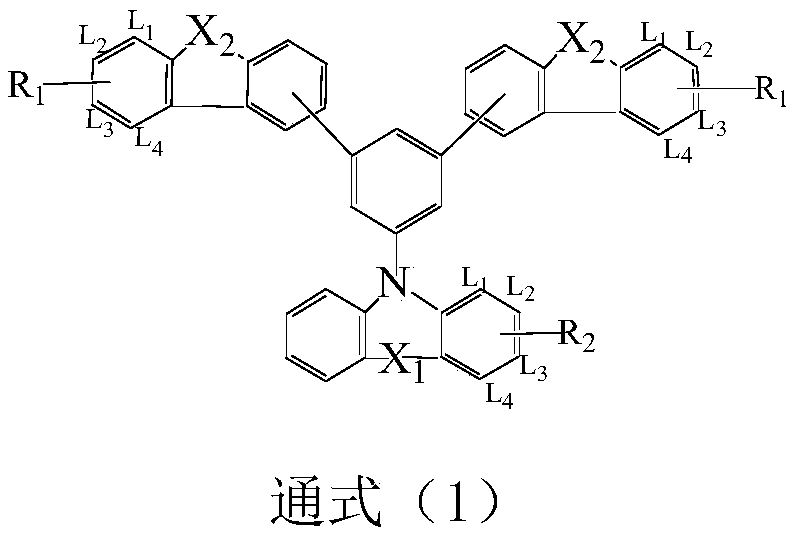
A compound with homobenzene as the core and its application in organic electroluminescent devices
A technology for light-emitting devices and compounds, applied in the fields of electric solid-state devices, organic chemistry, light-emitting materials, etc., can solve problems such as differences, and achieve the effect of improving triplet energy level, good application effect, and significantly improving device life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the synthesis of compound 9
[0046] (1) Synthesis of intermediates
[0047]
[0048] 3,5-Dibromochlorobenzene (2.7g, 10mmol), dibenzofuran-4-boronic acid (4.5g, 21mmol), sodium carbonate (10.2g, 96mmol), Pd 2 (dba) 3(0.4g, 0.4mmol), toluene, ethanol, and 50ml of water were successively added to the reaction flask, refluxed for 10 hours under nitrogen protection, cooled to room temperature, separated, the aqueous layer was extracted with ethyl acetate, and the organic layers were combined and used respectively Wash with saturated brine and water, dry the organic layer with magnesium sulfate, filter, spin the filtrate, and pass through a silica gel column to obtain 4 g of the product with a HPLC purity of 99.2%.
[0049] (2) Synthesis of compound 9
[0050]
[0051] Raw material S1 (2.2g, 5.0mmol), raw material S2 (1.5g, 5.1mmol) were added to the reaction flask, sodium tert-butoxide (1.0g, 10mmol), Pd 2 (dba) 3 (0.1g, 0.1mmol), 50ml of toluene w...
Embodiment 2
[0053] Embodiment 2: the synthesis of compound 23
[0054] (1) Synthesis of intermediates
[0055]
[0056] 3,5-Dibromochlorobenzene (2.7g, 10mmol), dibenzofuran-4-boronic acid (4.5g, 21mmol), sodium carbonate (10.2g, 96mmol), Pd 2 (dba) 3 (0.4g, 0.4mmol), toluene, ethanol, and 50ml of water were successively added to the reaction flask, refluxed for 10 hours under nitrogen protection, cooled to room temperature, separated, the aqueous layer was extracted with ethyl acetate, and the organic layers were combined and used respectively Wash with saturated brine and water, dry the organic layer with magnesium sulfate, filter, spin the filtrate, and pass through a silica gel column to obtain 4 g of the product with a HPLC purity of 99.2%.
[0057] (2) Synthesis of compound 23
[0058]
[0059] Raw material S1 (2.2g, 5.0mmol), raw material S3 (1.5g, 5.1mmol) were added to the reaction flask, sodium tert-butoxide (1.0g, 10mmol), Pd 2 (dba) 3 (0.1g, 0.1mmol), 50ml of toluen...
Embodiment 3
[0061] Embodiment 3: the synthesis of compound 33
[0062] (1) Synthesis of intermediates
[0063]
[0064] 3,5-Dibromochlorobenzene (2.7g, 10mmol), dibenzothiophene-4-boronic acid (4.8g, 21mmol), sodium carbonate (10.2g, 96mmol), Pd 2 (dba) 3 (0.4g, 0.4mmol), toluene, ethanol, and 50ml of water were successively added to the reaction flask, refluxed for 10 hours under nitrogen protection, cooled to room temperature, separated, the aqueous layer was extracted with ethyl acetate, and the organic layers were combined and used respectively Wash with saturated brine and water, dry the organic layer with magnesium sulfate, filter, spin the filtrate, and pass through a silica gel column to obtain 4.2 g of the product with a HPLC purity of 99.3%.
[0065] (2) Synthesis of compound 33
[0066]
[0067] Raw material S4 (2.4g, 5.0mmol), raw material S5 (1.4g, 5.1mmol) were added to the reaction flask, sodium tert-butoxide (1.0g, 10mmol), Pd 2 (dba) 3 (0.1g, 0.1mmol), 50ml of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com