Medicinal kallikrein and preparing method and application thereof
A technology of kininogenase and drugs, which is applied in the field of protein drugs, can solve the problems of inability to industrialize and the difficulty of separating and purifying kininogenase from porcine pancreas, and achieve the effects of small side effects, high biological activity and high drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Comparison of Purified KLK1 Single Component in Different Media
[0058] In order to purify the single-component sample in KLK1, the purification conditions were explored and a large number of media were screened. The specific results are shown in Table 1.
[0059] Table 1 Screening of different media
[0060] Media Name Purification yields the purity of single-component KLK1b Total protein yield CaptoQ ImpRes(GE) 80% 10% CaptoQ adhere(GE) 84% 10% DEAE Sepharose FF(GE) 50% 20% HP Q(GE) 98% 30% Q FF(GE) 90% 20% SOURCE Q(GE) 88% 10% Capto DEAE (GE) 60% 25% Super Q650(TOSH) 86% 18% Q-600AR(TOSH) 80% 15%
[0061] Through a large number of media screening results, it can be concluded that HP Q media (purchased from GE) has the best separation effect, the purity of single-component KLK1b can reach 98%, and the yield can reach 85%.
Embodiment 2
[0062] Example 2 Separation and purification of porcine pancreatic kininogenase single components KLK1b, KLK1a and KLK1c
preparation example 1
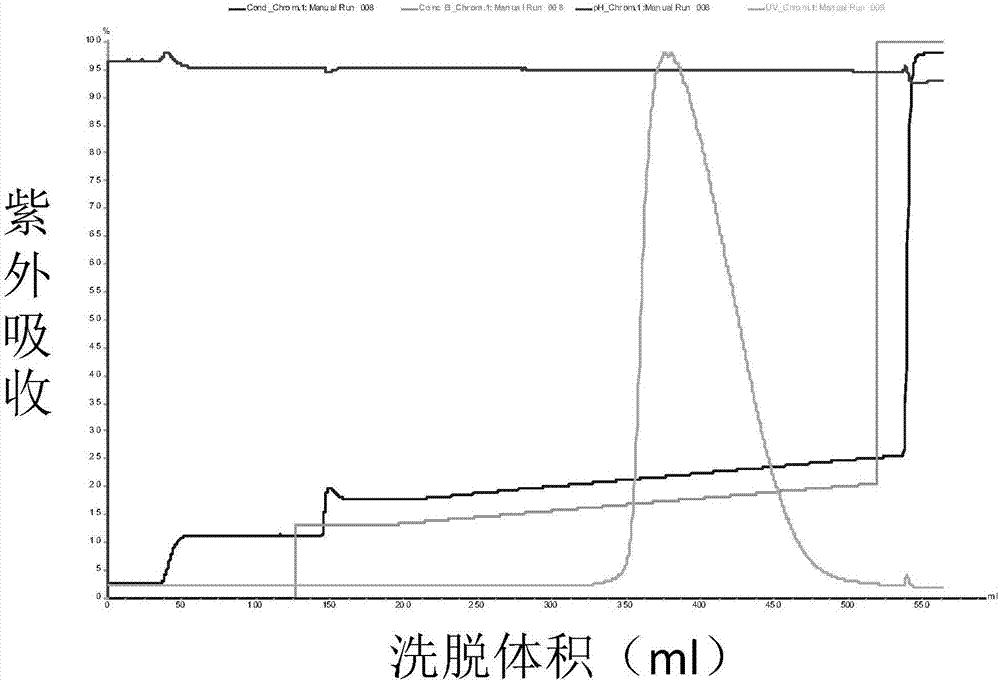
[0064] Porcine pancreatic kininogenase (from Changzhou Qianhong Biochemical Pharmaceutical Co., Ltd.) was diluted to 6 mg / mL with liquid A, and purified by ion-exchange chromatography. Purified chromatography conditions: ion exchange medium (QFF), A solution: 50mM Tris-HCl (pH9.0), B solution: 50mM Tris-HCl (pH9.0) containing 1M NaCl; flow rate 10mL / min, The detection wavelength is 280nm.
[0065] Loading: the above dilution of porcine pancreatic kininogenase was bound to a QFF ion exchange column.
[0066] Equilibration: Flush 5 column volumes with solution A.
[0067] Elution: The mobile phase ratio is 12% B, 88% A, after elution of 10 column volumes, gradient elution is performed. Gradient conditions were 12% B to 30% B, 30 column volumes.
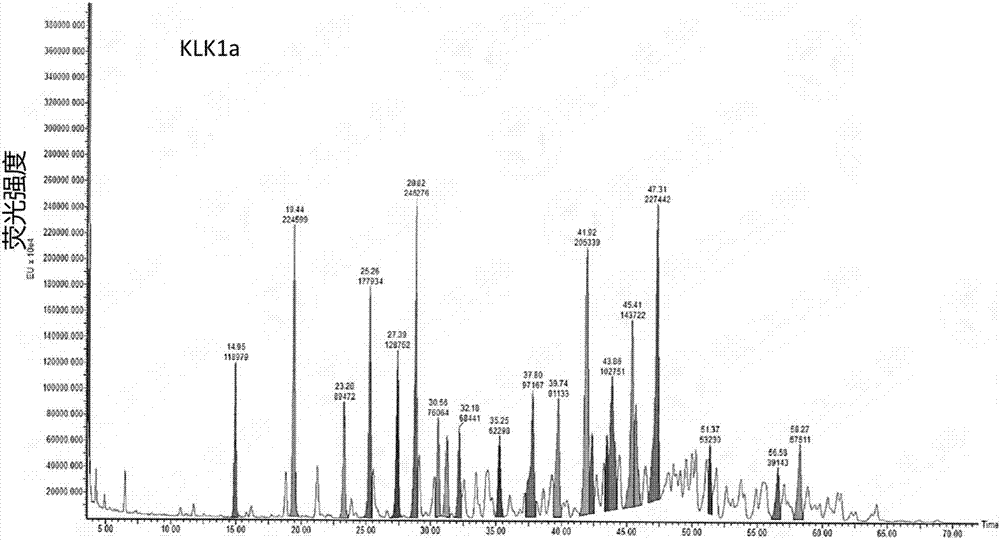
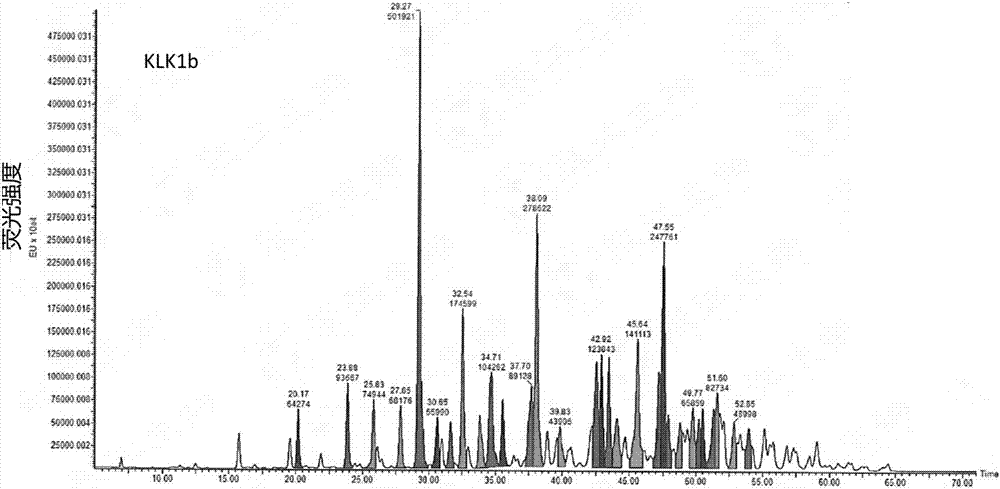
[0068] Collection: collect part of the eluted product before peak 1 as KLK1b, such as Figure 1a Shown; Partial elution product after peak 2 was collected as KLK1a, as Figure 2a As shown, the partially eluted product after peak 3 w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com