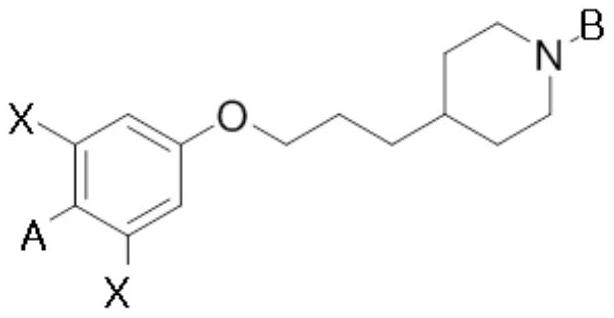
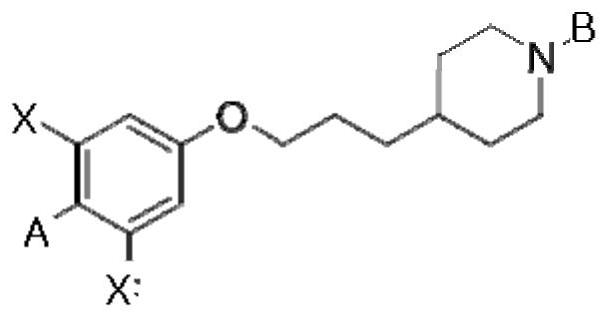
Compound having gpr119 agonist activity, method for preparing same, and pharmaceutical composition comprising same as effective component
A compound and pharmaceutical technology, applied in the field of compounds with GPR119 agonist activity, preparation of them and pharmaceutical compositions containing them as active components, can solve problems such as loss of curative effect and lack of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0207] (R)-5-(4-(3(3,5-difluoro-4-(4-methyl-4,5-dihydro Azol-2-yl)phenoxy)propyl)piperidin-1-yl)-3-isopropyl-1,2,4- Preparation of oxadiazole
[0208] (Step 1-1) Preparation of 4-(3-hydroxypropyl)piperidine-1-carbonitrile (chemical formula 3)
[0209]
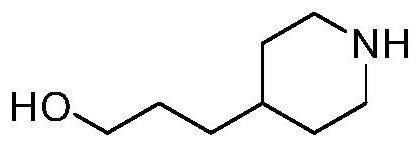
[0210] 3-(piperidin-4-yl)propan-1-ol hydrochloride (10g, 69.8mmol) of chemical formula 2 was dissolved in a mixed solution of dichloromethane (MC, 75.0ml) and water (55.0ml); Sodium bicarbonate (NaHCO 3 , 16.36g, 195.0mmol) was added thereto; then cyanogen bromide (6.48g, 61.2mmol) was added thereto; stirred at room temperature for 15 hours. An excess of aqueous ammonium chloride was added thereto; extracted with dichloromethane; then washed with brine. with MgSO 4 Moisture was removed from the organic layer, which was then filtered and concentrated under reduced pressure to obtain the desired form of compound 4-(3-hydroxypropyl)piperidine-1-carbonitrile in quantitative yield, which was used in the next step reactio...
preparation example 2
[0240] 3-(2,6-difluoro-4-(3-(1-(5-(5-isobutyl-1,2,4- Oxadiazol-3-yl)pyrimidin-2-yl)piperidin-4-yl)propoxy)phenyl)-5-methyl-1,2,4- Preparation of oxadiazole
[0241] (Step 2-1) Preparation of 3-(1-(5-bromopyrimidin-2-yl)piperidin-4-yl)propan-1-ol
[0242]
[0243] 3-(piperidin-4-yl)propan-1-ol (10g, 69.8mmol) and 5-bromo-2-chloropyrimidine (13.5g, 69.8mmol) of chemical formula 2 were dissolved in N,N-dimethyl Formamide (DMF, 10ml), then potassium carbonate (K 2 CO 3 , 10.6g, 76.8mmol) was added thereto, and the reaction was carried out at 80°C for 12 hours. The reaction solution was cooled to room temperature, diluted with water, extracted with ethyl acetate (EA, 150ml), and washed with brine. with MgSO 4 Water was removed from the organic layer, which was filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain the desired form of compound 3-(1-(5-bromopyrimidin-2-yl)piperidin-4-yl)propan-1-ol in 82% ...
preparation example 3
[0276] 2-(4-(3-(1-(5-ethylpyrimidin-2-yl)piperidin-4-yl)propoxy)-2,6-difluorophenyl)-5 -Methyl-1,3,4- Preparation of oxadiazole
[0277] (Step 3-1) Preparation of methyl 4-(3-(1-(5-ethylpyrimidin-2-yl)piperidin-4-yl)propoxy)-2,6-difluorobenzoate
[0278]
[0279] 2,6-difluoro-4-hydroxybenzoic acid methyl ester (1.72g, 9.16mmol) was dissolved in N,N-dimethylformamide (DMF, 30ml), and then 3-(1-(5- Ethylpyrimidin-2-yl)piperidin-4-yl)propyl methanesulfonate (3.3g, 10.08mmol) and potassium carbonate (K 2 CO 3 , 3.8g, 27.5mmol) was added to the reaction solution. The reaction solution was stirred at 65°C for 12 hours, then diluted with water and extracted with EA. with MgSO 4 Moisture was removed from the organic layer, the organic layer was filtered and concentrated under reduced pressure, and the residue was purified by silica gel column chromatography to obtain the desired form of compound 4-(3-(1-(5-ethyl pyrimidin-2-yl)piperidin-4-yl)propoxy)-2,6-difluorobenzoic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com