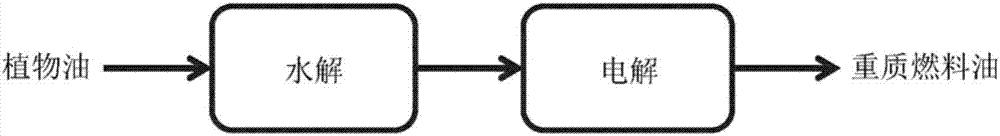
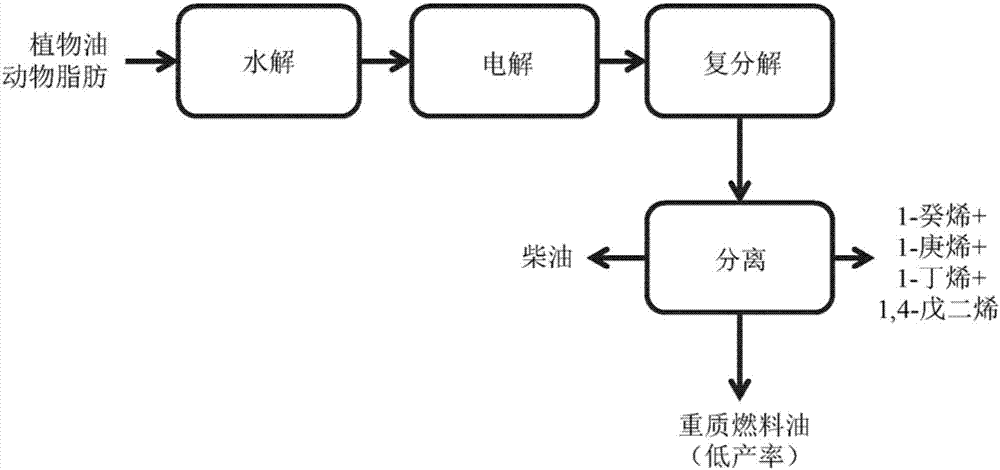
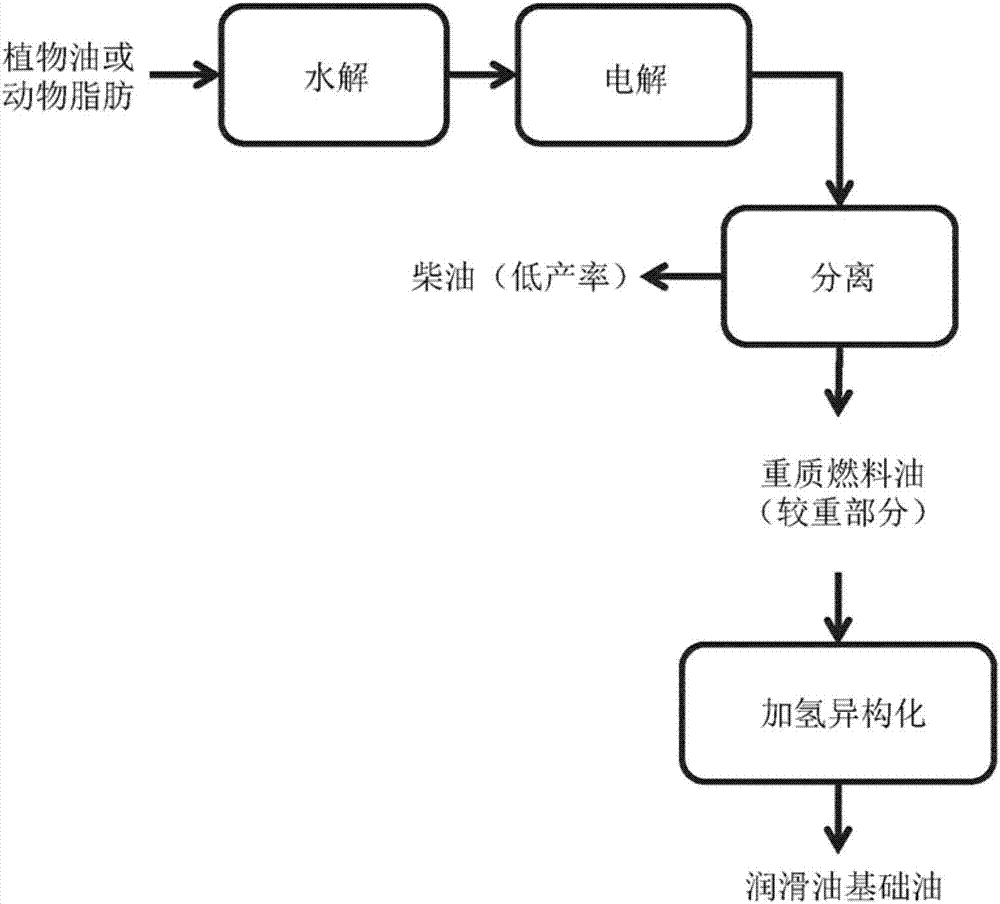
High productivity kolbe reaction process for transformation of fatty acids derived from plant oil and animal fat
A fatty acid, Colby technology, applied in the field of high productivity Colby reaction process, can solve problems such as reducing the yield of hydrocarbons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0078] Example 1 - Kolbe electrolysis reaction of fatty acids derived from tallow
[0079] 9.44 parts of fatty acid from tallow hydrolysis were added to 89.69 parts of methanol; 0.87 parts of potassium hydroxide was added to the mixture, which was then heated to 52°C in a water-jacketed vessel to give a clear solution. An electrolytic cell consisting of a platinum foil anode and a nickel cathode separated by a 1.5 mm spacing was immersed in the solution. A constant current density of 0.2A cm was used -2 . Within 1 hour, hydrocarbon products separated from the reaction mixture and accumulated at the bottom of the reactor, including coupled Kolbe electrolysis products and disproportionation products.
example 2
[0080] Example 2 - Kelby electrolysis reaction of oleic fatty acid in the presence of 0.6 wt% acetic acid based on total acid
[0081] 16.49 parts of oleic acid, 0.10 parts of acetic acid (0.6% by weight of total acids) and 0.98 parts of sodium hydroxide were dissolved in 59.47 parts of ethanol and 22.96 parts of water and heated to 50°C in a water-jacketed vessel. An electrolytic cell consisting of a platinum foil anode and a platinum foil cathode separated by a 1.5mm spacing was dipped into the solution. A constant current density of 0.1A cm was used -2 . After 50 minutes of electrolysis, an aliquot of the reaction mixture was acidified and extracted into hexane, then analyzed by gas chromatography, and the current yield and production rate were calculated (see Table 2).
example 3
[0082] Example 3 - Kolbe electrolysis reaction of oleic fatty acid at moderate current density
[0083] 16.26 parts of oleic acid and 1.15 parts of sodium hydroxide were dissolved in 53.52 parts of ethanol and 29.07 parts of water and heated to 50°C in a water-jacketed vessel. An electrolytic cell consisting of a platinum foil anode and a platinum foil cathode separated by a 1.5mm spacing was dipped into the solution. A constant current density of 0.1A cm was used -2 . After 100 minutes of electrolysis, an aliquot of the reaction mixture was acidified and extracted into hexane, then analyzed by gas chromatography and the current yield and production rate were calculated (see Table 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com