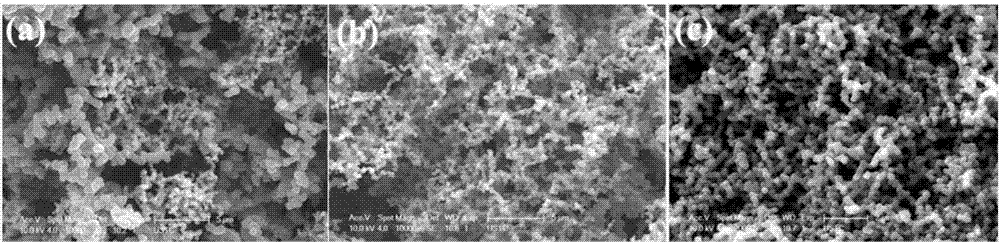
Efficient compounding method for chain-like carbon nanospheres
A technology of carbon nanospheres and synthesis methods, applied in the direction of nanocarbons, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of small yield, high cost, low purity, etc., and achieve The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
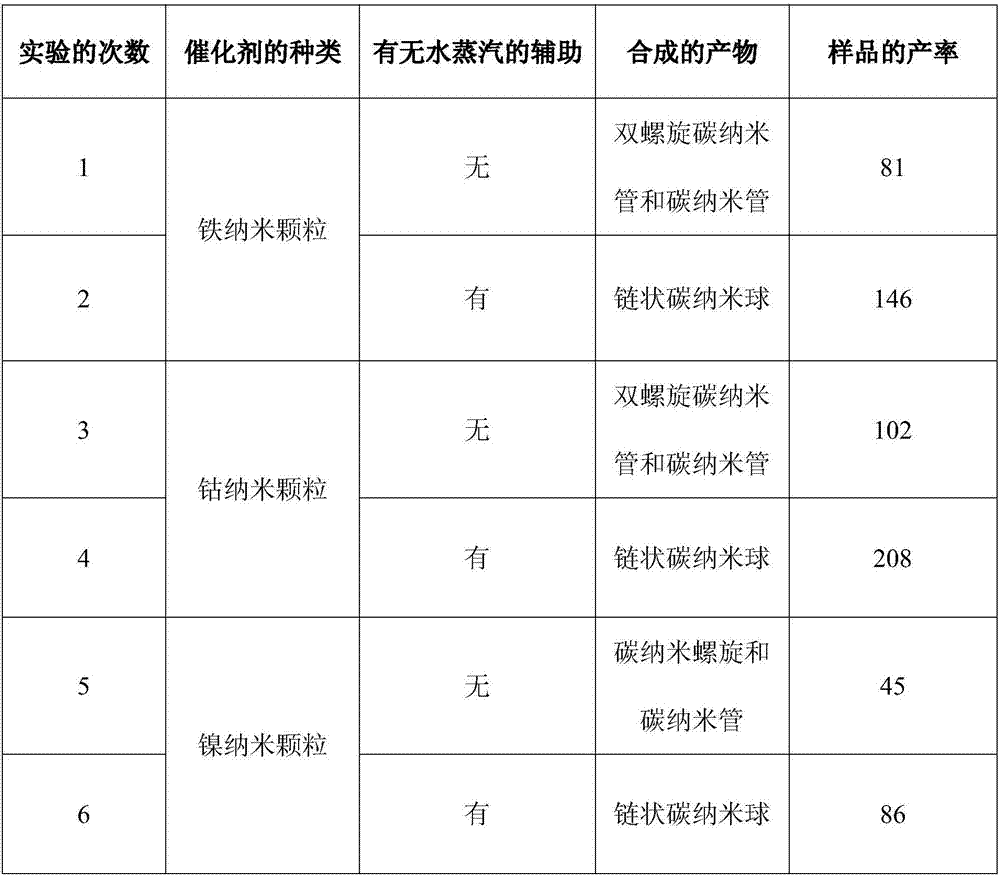
[0012] Embodiment 1: the first step: at first 1mol FeCl 2 4H 2 0. 1.5mol of citric acid particles were mixed, 100mL of absolute ethanol was added thereto, and the resulting mixture was put into a three-necked bottle and stirred in a water bath at 60°C for 6 hours, then the resulting sol was heat-treated at 80°C in an oven for 6 hours, and then heated to Heat treatment at 150°C until the corresponding xerogel is obtained; heat-treat the obtained xerogel in a high-temperature furnace at 450°C in air for 4 hours to obtain the desired catalyst precursor powder (iron oxide nanoparticles);
[0013] Step 2: Take 50 mg of the synthesized catalyst precursor, spread it in a small porcelain boat and push it into the tubular quartz tube to the position of the thermocouple of the tubular furnace, and raise the reaction temperature from room temperature under the protection of argon. up to 450°C; then turn off the argon and immediately switch to the hydrogen, and reduce the obtained cataly...
Embodiment 2
[0015] CoCl 2 ·6H 2 O was mixed with citric acid particles and absolute ethanol according to the quantity ratio of 1mol:1.5mol:100mL, and after stirring in a water bath at 70°C for 7 hours, the obtained sol was heat-treated at 60°C in an oven for 8 hours, and then heated to 120°C for heat treatment until The corresponding xerogel was obtained; (2) the obtained xerogel was heat-treated at 500°C in the air for 5 hours to obtain the desired catalyst precursor powder; (3) a certain amount of catalyst precursor powder was spread on the small porcelain In the boat, under the protection of argon, the reaction temperature is raised from room temperature to 450°C, the argon is turned off and the hydrogen gas is switched immediately, and the catalyst precursor obtained is reduced by hydrogen at the 450°C for 1-4 hours (4) Warm up to 550-600°C under the protection of argon, and immediately switch to acetylene and water vapor for 2 hours. After cooling to room temperature, a large number...
Embodiment 3
[0017] (1) NiCl 2 ·6H 2 O was mixed with citric acid particles and absolute ethanol according to the quantity ratio of 1mol:1.5mol:100mL, and after stirring in a water bath at 50°C for 5 hours, the obtained sol was heat-treated at 70°C in an oven for 5 hours, and then heated to 130°C for heat treatment until The corresponding xerogel was obtained; (2) the obtained xerogel was heat-treated at 430°C in the air for 3 hours to obtain the desired catalyst precursor powder; (3) a certain amount of catalyst precursor powder was spread on the small porcelain In the boat, under the protection of argon, the reaction temperature is raised from room temperature to a specific temperature of 375°C, the argon is turned off and the hydrogen gas is switched immediately, and the obtained catalyst precursor is reduced by hydrogen at the specific temperature for 1-4 (4) under the protection of argon, the temperature was raised to 550-600° C., and acetylene and water vapor were immediately switch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com