Method for preparing pranlukast key intermediate 3-amino-2-hydroxyacetophenone
A technology for hydroxyacetophenone and intermediates, applied in the field of preparation of 3-amino-2-hydroxyacetophenone, the key intermediate of pranlukast, can solve the problems of low yield and long synthesis route, and achieve The effect of high purity, novel synthetic route, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
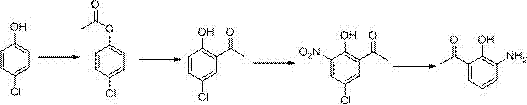
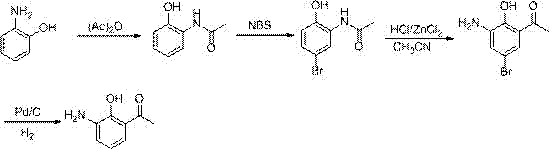
[0041] The first step: the preparation of 2-acetamidophenol
[0042] Take 2-aminophenol (109kg), dissolve it in 250kg of water, add acetic anhydride (110kg) dropwise under ice bath, after the dropwise addition, warm up to room temperature and react for 2h. After the reaction, filter to obtain 142kg of light yellow solid, collect The rate is 94%.
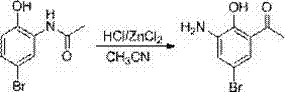
[0043] The second step: the preparation of 2-acetamido-4-bromophenol
[0044] Take 75kg of 2-acetaminophen, dissolve it in 200kg of dichloromethane, cool in an ice bath to 0°C, add 90kg of NBS in batches, then warm up to room temperature and react for 3 hours. During the reaction, solids are gradually precipitated. After the reaction, filter and filter The cake was washed with 500kg of water, and dried to obtain 104kg of yellow solid powder, with a yield of 91%.
[0045] Step 3: Preparation of 2-hydroxy-3-amino-5-bromoacetophenone
[0046]Get 114kg of 2-acetamido-4-bromophenol, dissolve it in 200kg of acetonitrile, add 10kg of ZnC...
Embodiment 2
[0050] The first step: the preparation of 2-acetamidophenol
[0051] Take 2-aminophenol (110kg), dissolve it in 255kg of water, add acetic anhydride (112kg) dropwise under ice bath, after the dropwise addition, warm up to room temperature and react for 2h. After the reaction, filter to obtain 145kg of light yellow solid, collect The rate is 94%.
[0052] The second step: the preparation of 2-acetamido-4-bromophenol
[0053] Take 78kg of 2-acetaminophen, dissolve it in 210kg of dichloromethane, cool it in an ice bath to 0°C, add 92kg of NBS in batches, then raise the temperature to room temperature and react for 3 hours. During the reaction, solids are gradually precipitated. After the reaction, filter and filter The cake was washed with 500kg of water, and dried to obtain 106kg of yellow solid powder, with a yield of 91%.
[0054] Step 3: Preparation of 2-hydroxy-3-amino-5-bromoacetophenone
[0055] Get 115kg of 2-acetamido-4-bromophenol, dissolve it in 210kg of acetonitril...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com