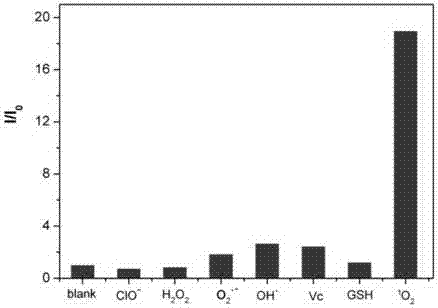
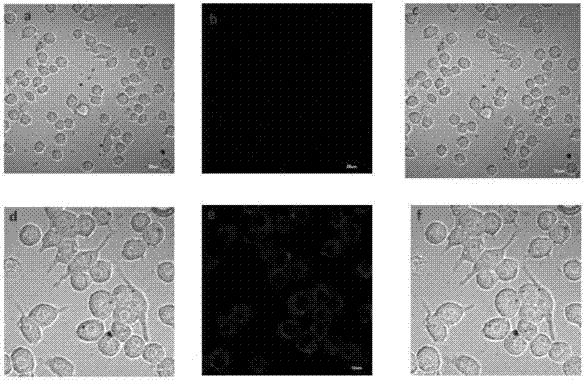
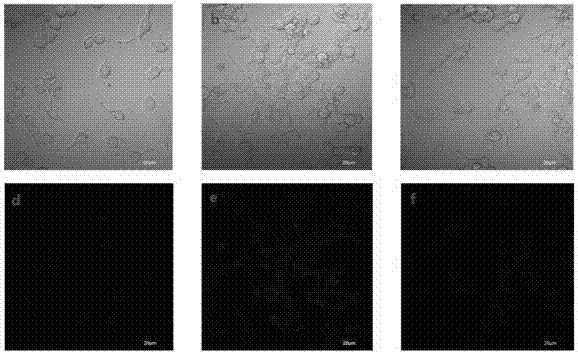
Dye and fluorescent probe for detecting singlet oxygen and manufacturing method of dye and fluorescent probe
A fluorescent probe and singlet oxygen technology, applied in chemical instruments and methods, fluorescence/phosphorescence, dibenzoanthone/isodibenzoanthrone dyes, etc., can solve highly sensitive and selective fluorescence quenching Solve the problems of scarcity of reagents, difficulty in synthesis, and low sensitivity, and achieve the effects of reducing synthesis cost, expanding application range, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the synthesis of formula I probe
[0053] Such as figure 1 As shown, using 9,10-dioxo-9,10-dihydroanthracene-1,4-dicarboxychloride (for example, as shown in CAS No. 6937-78-6) as a raw material, the compound of formula I was prepared and synthesized. Alternatively, in order to obtain 9,10-dioxo-9,10-dihydroanthracene-1,4-dicarboxychloride, commercially available or synthetic 9,10-dioxo - 9,10-dihydroanthracene-1,4-dicarboxylic acid (eg, CAS No. 54292-11-4), subjecting it to a chlorination reaction with a chlorinating agent (eg, thionyl chloride).
[0054] For example, 9,10-dioxo-9,10-dihydroanthracene-1,4-dicarboxylic acid can be synthesized first: prepare 1,4- Dimethylanthraquinone (1.3g, 5.51mmol) suspension, add active MnO in batches while stirring at 80°C 2 (6.88 g, 79.1 mmol). After 15 minutes, heat to 110°C and hold for 4 hours. Then, after the reaction mixture had cooled, it was slowly poured into cold water. The resulting solid was isolated, ...
Embodiment 2
[0064] Embodiment 2: the piperazine derivative of formula I compound is synthesized
[0065] Such as figure 2 As shown, piperazine derivatives of compounds of formula I were prepared. For example, piperazine (5.31mg, 0.062mmol) was dissolved in dry dichloromethane (DCM) (6mL), then benzotriazole-N,N,N',N'-tetramethyluronium hexafluoro Phosphate (HBTU) 34.3mg, 0.091mmol), N, N-diisopropylethylamine (DIPEA) (31μl, 0.184mmol), and compound 1 (34mg, 0.062mmol) stirred overnight at room temperature, namely The crude product of the piperazine derivative of the compound of formula (I) (ie compound 5) was obtained. The product can be purified with HCl (1M, 10mL) and NaHCO 3 Aqueous solution (2X 10 mL) washed the reaction mixture. The organic phase was collected and rotovaped to dryness. By silica gel column chromatography (eluent: CH 2 Cl 2 / MeOH, v / v=97: 3)) purify the crude product to obtain pure purple piperazine derivatives of the compound of formula (I) (wherein R 1 is m...
Embodiment 3
[0070] Embodiment 3: the synthesis of formula IIa singlet oxygen probe
[0071] Such as figure 2 As shown, according to the following steps, the singlet oxygen fluorescence detection probe of formula IIa was synthesized.
[0072] Synthesis of Compound 3: According to the description in Sunahara et al. 2007 (Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T.J.AM.CHEM.SOC.2007, 129, 5597-5604), the compound was prepared 3. Its chemical structure is as figure 2 Compound 3 in.
[0073] Synthesis of Compound 4 : To a solution of compound 3 (35 mg, 0.10 mmol) in anhydrous dichloromethane (8 mL) was added chloroacetyl chloride (30 μL, 0.41 mmol, dissolved in 1 mL of dichloromethane) and triethylamine (30 μL, 0.215 mmol). Stir the reaction at room temperature for 2 hours, then wash with NaHCO 3 The reaction mixture was washed with aqueous solution (3 x 10 mL) and deionized water (3 x 10 mL). The organic phase was collected and rotovaped to dryness, then used further in the next s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com