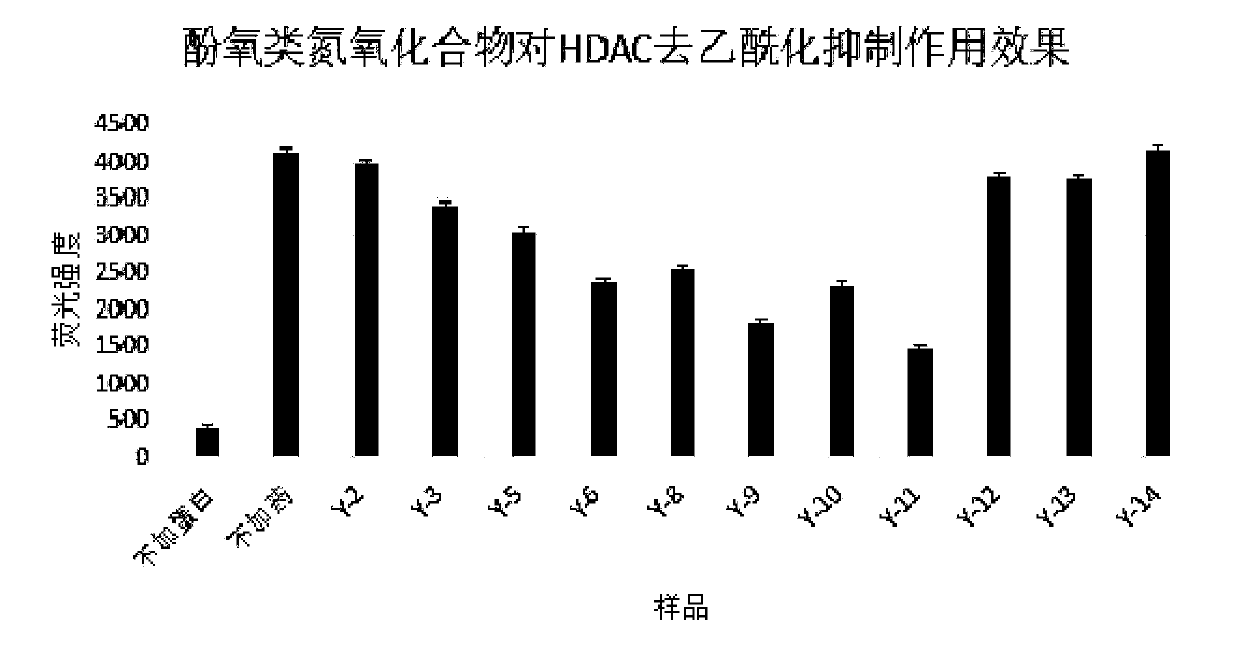
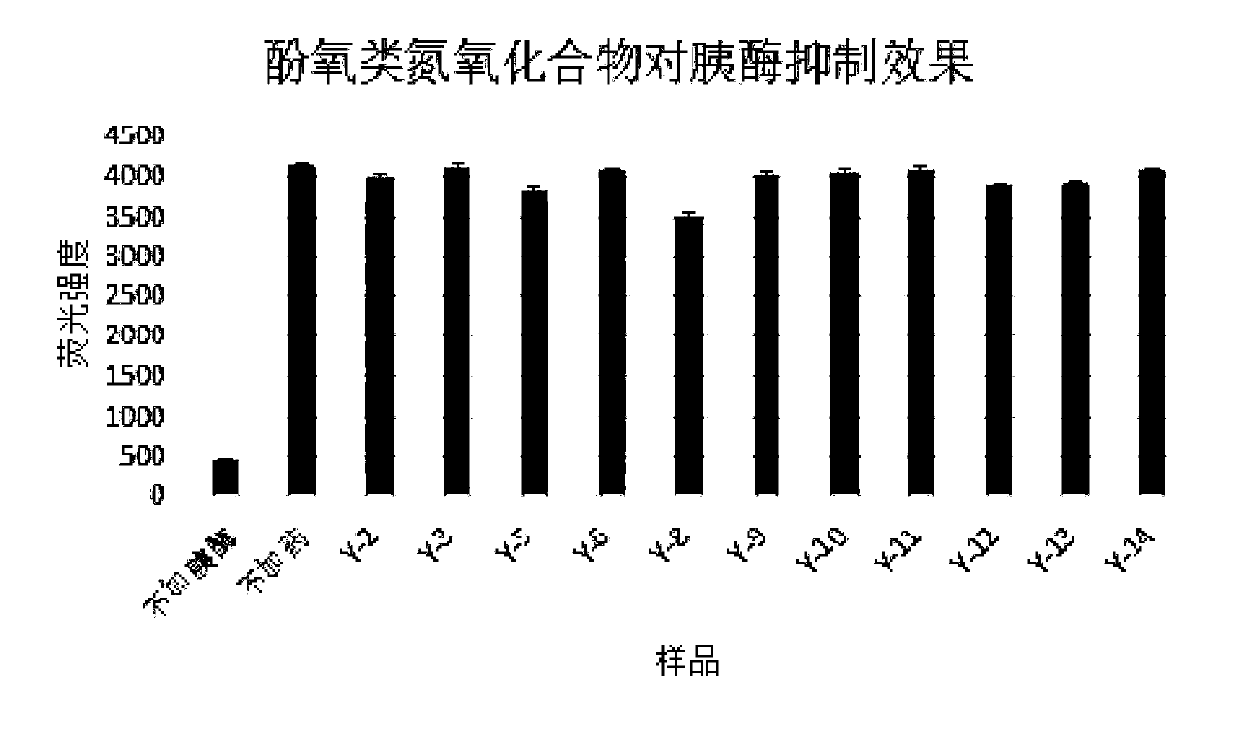
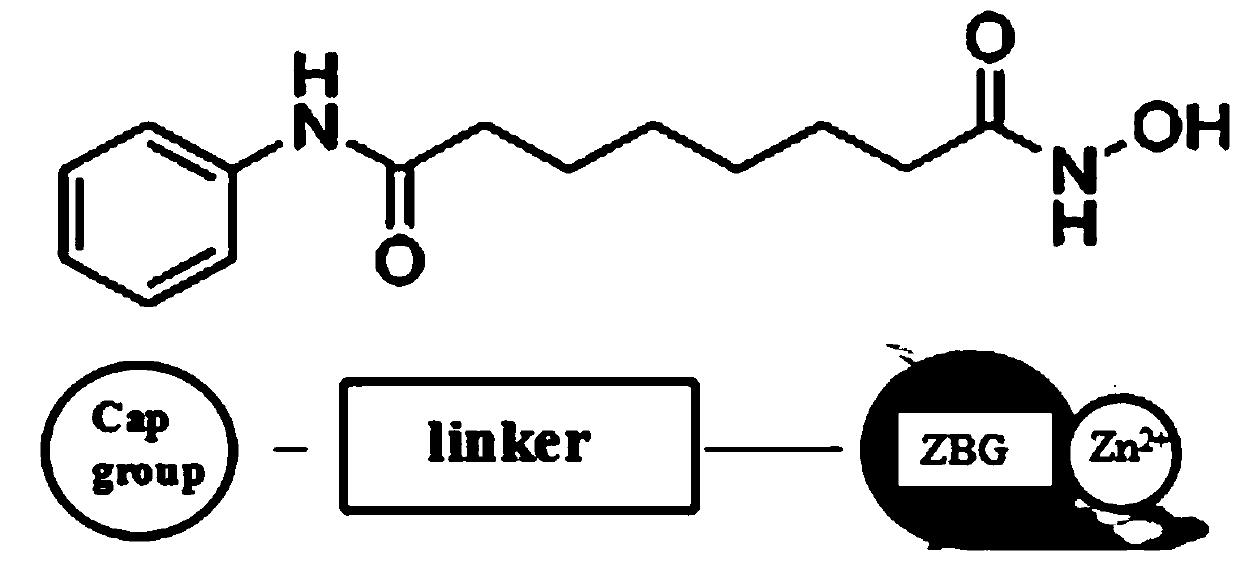
Antitumor application of phenolic nitrogen oxides as sirtuin inhibitors
A sirtuin, nitrogen oxide technology, applied in the field of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The technical solution of the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments.
[0031] 1. Synthesis of Compound Y-1:
[0032]
[0033] Take a 100mL eggplant-shaped bottle and dry it, weigh p-hydroxybenzoic acid (1160mg, 8.40mmol), add tetrahydrofuran (THF, 10mL) to dissolve, stir, add aniline (PhNH 2 , 1mL) and thionyl chloride (SOCl 2 ,0.874mL) after 0.5h, put it at room temperature to react for 26h, the reaction was completed, concentrated, and the silica gel column was eluted with dichloromethane:methanol=60:1,50:1,40:1 gradient to obtain white powder Y-1 312mg , the yield is 26.9% 1 H-NMR (400MHz, DMSO) δ (ppm): 7.80-7.77 (m, 2H) 7.61-7.59 (d, 2H, J = 8Hz) 7.37-7.33 (m, 2H) 7.14-7.11 (d, 1H) 6.91 -6.89(d,2H,J=8Hz); 13 C-NMR (300Hz, DMSO) δ (ppm): 165.03, 160.49, 139.43, 128.41, 125.35, 123.14, 120.20, 114.81.
[0034] 2. Synthesis of Compound Y-2:
[0035]
[0036] Take Y-1 (300mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com