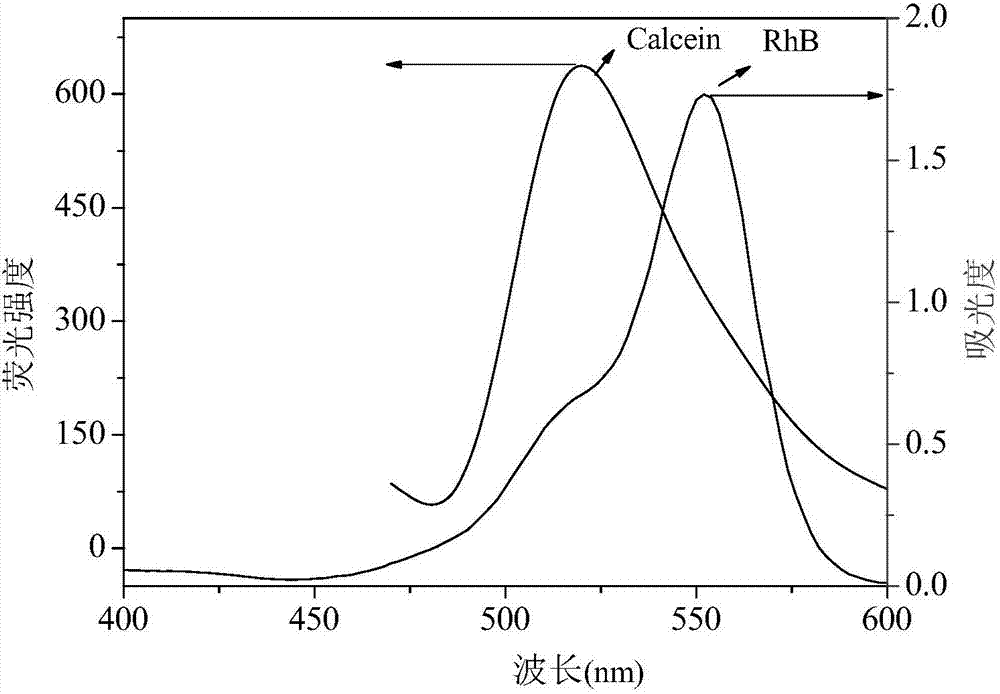
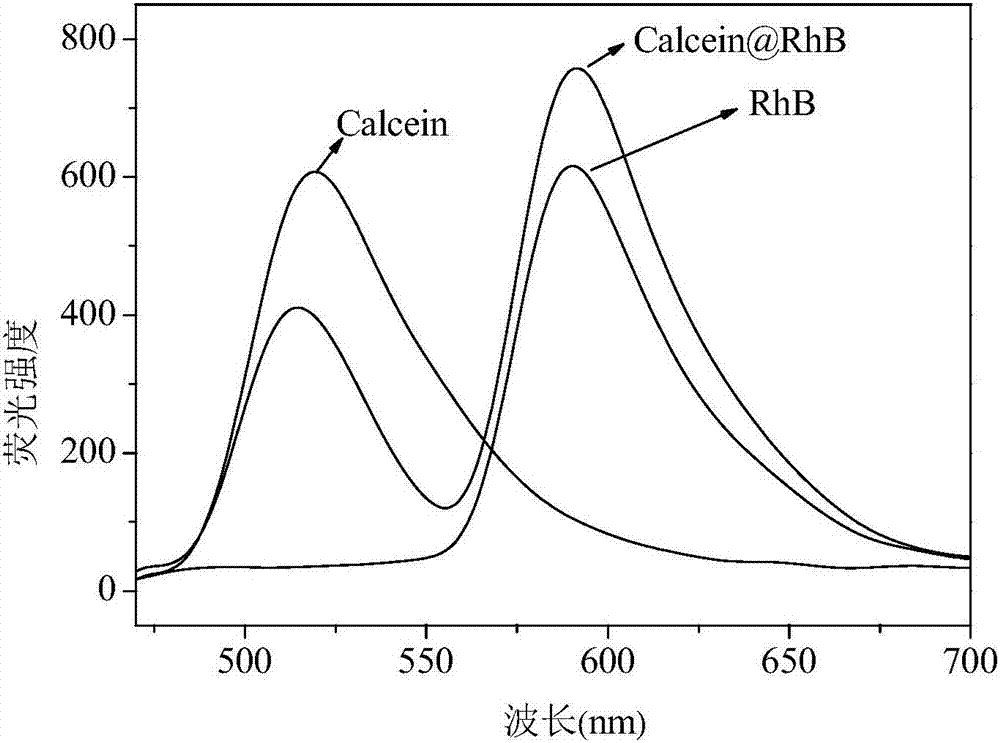
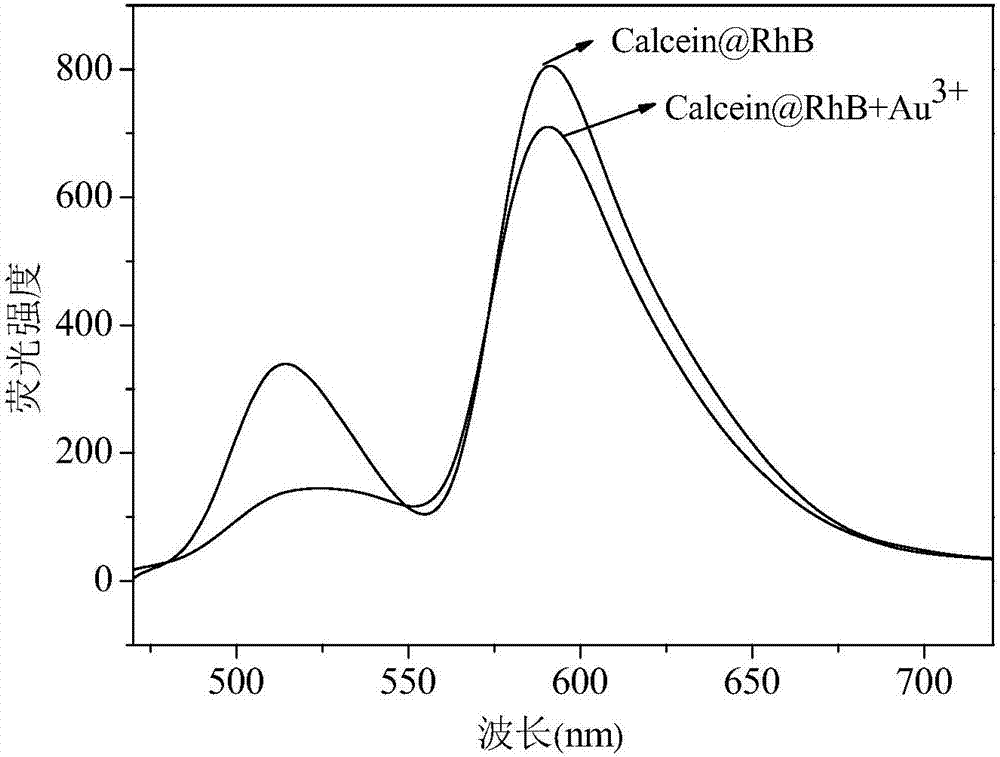
Method for detecting S<2> based on calcein-rhodamine B-gold ion system
A technology of calcein and gold ions, applied in the field of detection of S2-based on calcein-rhodamine B-gold ion system, can solve the problems of low sensitivity, high cost, time-consuming, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A Calcein-Rhodamine B-Au(III) System Based on the Detection of S 2- The method, described method comprises the following steps:
[0043] (1) Take 100μL of 10 -5 mol / L of calcein and 1000μL 10 -4 mol / L rhodamine B in 10mL test tube, add 200μL 10 -4 mol / L chloroauric acid solution, then add 100 μL of PBS buffer solution, adjust the pH to 5.9, stir and react for 25 minutes, and prepare the aqueous solution of calcein-rhodamine B-Au(III);
[0044] (2) Add 0 μL, 1 μL, 8 μL, 30 μL, 50 μL, 80 μL, 130 μL, 160 μL, 190 μL, 220 μL, 240 μL, 260 μL to the above-mentioned aqueous solution of calcein-rhodamine B-Au(III) at a concentration of 10 -3 mol / L of S 2- solution, diluted to 10 mL with deionized water, to obtain S 2- The final concentrations of the solutions were 0, 0.1 μmol / L, 0.8 μmol / L, 3 μmol / L, 5 μmol / L, 8 μmol / L, 13 μmol / L, 16 μmol / L, 19 μmol / L, 22 μmol / L, 24 μmol / L, 26μmol / L, mixed and stirred for 25min to obtain the detection system;
[0045] (3) The excitation w...
Embodiment 2
[0049] Calcein@RhB / Au(III) system to S 2- selective experiment
[0050] Add some other common anions, such as SO 3 2- 、HPO 4 2- 、Ac - , NO 2 - , F - , SO 4 2- , NO 3 - , Cl - , I - , CO 3 2- For the selectivity test, measure the fluorescence intensity, and make a histogram with (F0-F) / F0 as the ordinate and the ion species as the abscissa, and then add these ions to the Calcein@RhB / Au(III) system Add S to 2- , measure its fluorescence intensity, and make a histogram with (F0-F) / F0 as the ordinate and ion species as the abscissa. In this test, the final concentration of other anions is 120 μmol / L, S 2- The final concentration was 6 μmol / L, and other experimental conditions were the same as in Example 1.
[0051] The result is as Figure 9 It can be seen from the figure that when other anions of the same concentration are added to the Calcein@RhB / Au(III) fluorescent system, the fluorescence intensity of the Calcein@RhB / Au(III) system is quenched by other anion...
Embodiment 3
[0053] Influence of Reaction Time on the Fluorescence Intensity of the System
[0054] The reaction time t is one of the very important parameters, and the reaction time t will affect the sensitivity of target analyte detection. In the case that other conditions are the same as in Example 1, S 2- The final concentration was 1 μmol L -1 , change the reaction time of steps (1) and (2), and test the change of the fluorescence intensity of the system under different reaction times, the results are as follows Figure 10 As shown, it can be seen from the figure that when the reaction reaches 25 minutes, the fluorescence intensity of the system tends to be stable, so the reaction time of the control system in the present invention is 25 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com