Method for preparing capacitor electrode material non-stoichiometric lanthanum manganate
A technology for stoichiometric lanthanum manganate and electrode materials, applied in hybrid capacitor electrodes, nanotechnology for materials and surface science, manganate/permanganate, etc. Time and other issues, to achieve the effect of low cost, good material performance, high cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
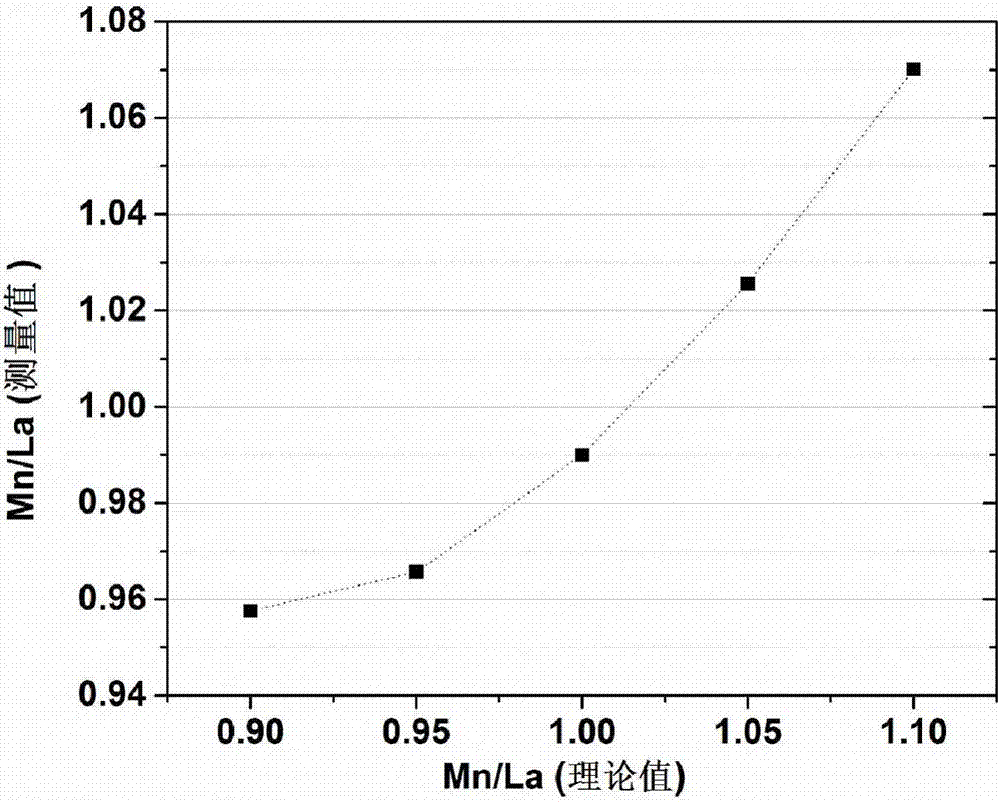
[0026] (1) Weigh 26.846g of lanthanum nitrate hexahydrate and add it to 20mL of water under the action of magnetic stirring. Then, 12.2044 g of manganese nitrate (Mn / La molar ratio=1.1) was weighed and added to the above solution, and stirred for 2 h to ensure that a homogeneously mixed nitrate mixture was obtained. Then 10.4 mL of citric acid (15.5 mol / L) was added dropwise.
[0027] (2) The above mixed solution was placed in a water bath at 80°C, vigorously stirred for 4 hours, and the excess water was evaporated until a brown gel was obtained. The obtained sol-gel was transferred to a vacuum drying oven and dried at 140 °C for 8 h.
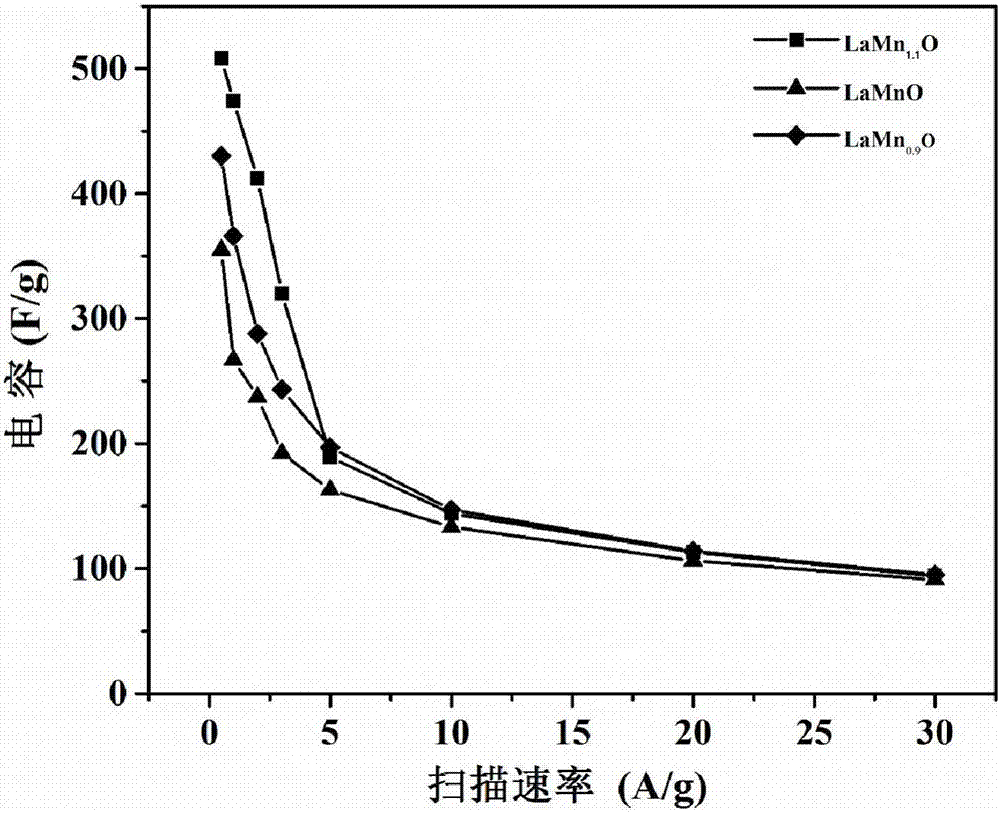
[0028] (3) The xerogel obtained in the above steps was put into an agate mortar and ground, then transferred to a tube furnace, and preheated to 350° C. for 10 minutes. The burnt powder was ground and calcined at 750°C for 4h in a tube furnace in an air atmosphere. Get LaMn 1.1 O 3 .
Embodiment 2
[0030] (1) Weigh 26.846g of lanthanum nitrate hexahydrate and add it to 20mL of water under the action of magnetic stirring. Then, 11.6496 g of manganese nitrate (Mn / La molar ratio=1.05) was weighed and added to the above solution, and stirred for 2 h to ensure that a uniformly mixed nitrate mixture was obtained. Then 10.2 mL of citric acid (15.5 mol / L) was added dropwise.
[0031] (2) The above mixed solution was placed in a water bath at 80°C, vigorously stirred for 4 hours, and the excess water was evaporated until a brown gel was obtained. The obtained sol-gel was transferred to a vacuum drying oven and dried at 140 °C for 8 h.
[0032] (3) The xerogel obtained in the above steps was put into an agate mortar and ground, then transferred to a tube furnace, and preheated to 350° C. for 10 minutes. The burnt powder was ground and calcined at 750°C for 4h in a tube furnace in an air atmosphere. Get LaMn 1.05 O 3 .
Embodiment 3
[0034] (1) Weigh 26.846g of lanthanum nitrate hexahydrate and add it to 20mL of water under the action of magnetic stirring. Then, 11.0949 g of manganese nitrate (Mn / La molar ratio=1.00) was weighed and added to the above solution, and stirred for 2 h to ensure that a homogeneously mixed nitrate mixture was obtained. Then 10.0 mL of citric acid (15.5 mol / L) was added dropwise.
[0035] (2) The above mixed solution was placed in a water bath at 80°C, vigorously stirred for 4 hours, and the excess water was evaporated until a brown gel was obtained. The obtained sol-gel was transferred to a vacuum drying oven and dried at 140 °C for 8 h.
[0036] (3) The xerogel obtained in the above steps was put into an agate mortar and ground, then transferred to a tube furnace, and preheated to 350° C. for 10 minutes. The burnt powder was ground and calcined at 750°C for 4h in a tube furnace in an air atmosphere. Get LaMnO 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com