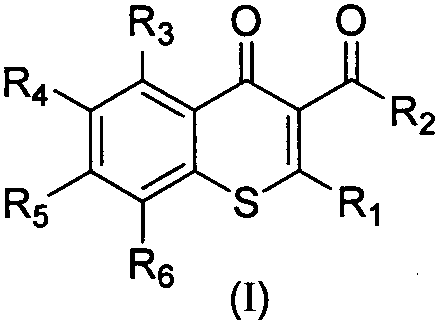
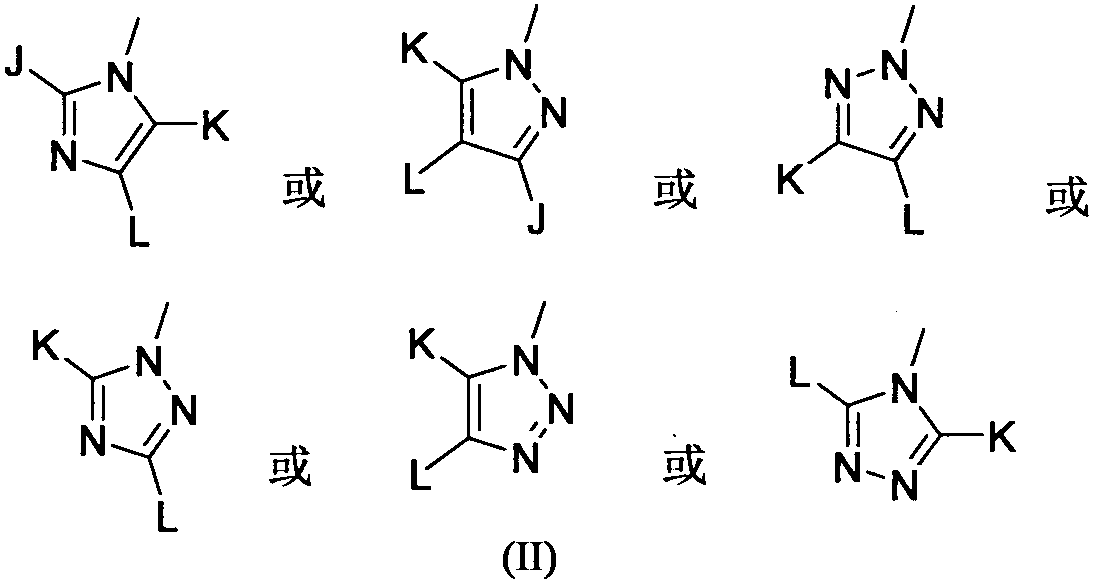
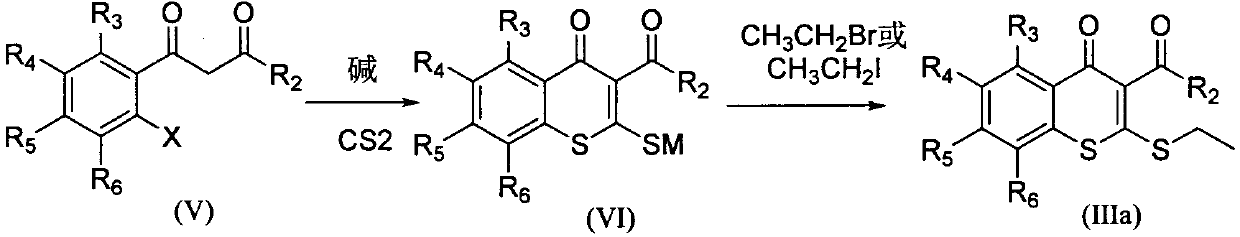
A kind of 2-oxazothione compound and its synthesis method and its application in the preparation of antifungal medicine
The technology of a compound, thiochromone, is applied in the field of preparation of antifungal drugs, which can solve the problems of narrow antifungal spectrum, narrow antifungal broad spectrum and weak in vivo activity of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the synthetic preparation reaction formula of formula III1 is as follows:
[0051]
[0052] Follow the steps to prepare:
[0053] Add 22.4mmol of methyl 2,4-dichloro-5fluorobenzoylacetate, 30mL of DMSO, and 45mmol of NaOH into a single-necked flask, heat up to 40°C under stirring, and add CS dropwise at this temperature 2 26.3 mmol in 5 mL DMSO. After dropping, the temperature was controlled at 40°C and the reaction was stirred. After the reaction is complete, cool to room temperature, add 26.3 mmol of iodoethane in 10 mL of DMSO solution dropwise, after the drop is complete, react at room temperature until the reaction is complete, stir the reaction solution and pour it into 50 mL of ice water, a large amount of solids are precipitated, collect the solids by filtration, and put them in ethanol After recrystallization, 16.8 mmol of methyl 2-ethylthio-6-fluoro-7-chlorothiochromone-3-carboxylate, a product of formula (III1), was obtained, with a yield ...
Embodiment 2- Embodiment 20
[0055] Embodiment 2-embodiment 20: the preparation of formula (III) thiochromone compound
[0056] With (IV) compound as raw material, prepare product formula (III) compound (target product is each compound of formula (III2)~(III15) in table 1), preparation step is the same as embodiment 1, and reaction formula is as follows:
[0057]
[0058] In Example 2 to Example 15, the selection of each group of the product formula (III) thiochromones and the preparation reagents and detection data are listed in Table 1.
[0059] Table 1
[0060]
[0061]
[0062]
[0063]
Embodiment 16
[0064] Embodiment 16: Synthesis and preparation of formula III16 The reaction formula is as follows:
[0065]
[0066] Prepare as follows:
[0067] Into a 100mL single-necked flask, add 10mmol of 2-ethylthio-6-fluoro-7-chlorothiochromone-3-carboxylate, 20mL of acetic acid in sequence, heat to 65°C, and dropwise add 30% H 2 o 2 12mmol was stirred and reacted for 6 hours, and sodium sulfite was added to extract it, and the solvent acetic acid was distilled off under reduced pressure, then extracted with 50mL dichloromethane and 50mL water, the dichloromethane layer was taken, washed twice with water (50mL×2), the organic layer was recovered, and the The product of formula (III16) was distilled to obtain 9.5 mmol of methyl 2-ethanethionyl-6-fluoro-7-chlorothiochromone-3-carboxylate, with a yield of 95%.
[0068] 1 HNMR (DMSO-d 6 ): 1.28(t, 3H), 3.06-3.18(m, 1H), 3.31-3.43(m, 1H), 3.87(s, 3H), 8.16(d, 1H), 8.60(d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com