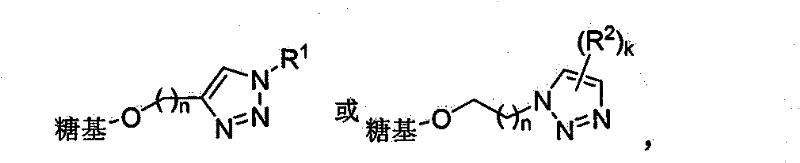
Triazole glucoside, method for synthesizing same by enzyme chemical method and application thereof
A triazole glucoside and glucosidase technology, applied in organic chemistry, medical preparations containing active ingredients, sugar derivatives, etc., can solve the problems of slow development of new antifungal drugs, and achieve simple preparation methods and high product activity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 contains the glucoside synthesis of alkynyl / azide
[0049] 1.1 Preparation of glycosidase powder
[0050] Soak peach kernels or apple seeds in water for 2 hours, select intact and non-mildewed seeds, remove the skin, wash the floating water on the surface of the seeds with twice the volume of acetone, grind them with a tissue grinder in the presence of ethyl acetate, and use ethyl acetate Degreasing three times, acetone degreasing and dehydration twice. Enzyme powder is stored in a 4C freezer for future use.
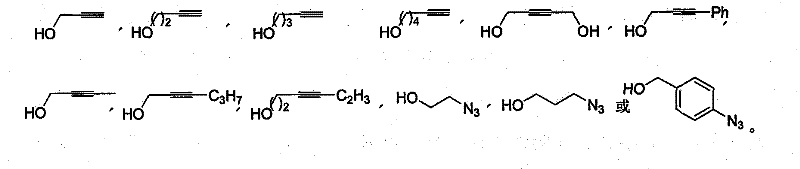
[0051] 1.2 General Synthetic Method of Alkyne / Azide-Containing Glucosides
[0052]Add 0.54 g of glucose to a 25 ml egg bottle, dissolve in 1 ml of water, inject 5 ml of alkynyl / azido alcohol, 5 ml of acetonitrile, add 0.6 g of malosidase or myranosidase, seal with an inversion plug, pump vacuum, and argon Protected by a balloon, stirred at 50°C for 72 hours, filtered with silica gel, washed with methanol (5 ml X4), evaporated to remove the solvent ...
Embodiment 2
[0075] Example 2 Synthesis of galactosides containing alkynyl / azide
[0076] 2.1 General method for the synthesis of galactoside by transglycosidation
[0077] 2.5 g lactose was dissolved in 5 ml buffer solution (sodium acetate, 20 mM, pH 4.5) and propargyl alcohol was added. Add 0.1 g (900 U) of Aspergillus oryzae galactosidase to the system and stir at room temperature for 12 hours at 25°C. The reaction solution was filtered to remove enzymes and lactose, and the filtrate was vacuum-concentrated to a light yellow syrup, dissolved in methanol, adsorbed on crude silica gel, and subjected to column chromatography (ethyl acetate / methanol=15~10 / 1 or dichloromethane / methanol=5 / 1) elute. The target compound galactoside was obtained.
[0078] 2.1 Spectral data of alkyne / azide-containing galactosides
[0079] 2-Alkyne-propyl β-galactoside (3a):
[0080] white solid; [α] 26 D -30.2 (c0.71, CH 3 OH); 1 H NMR (300MHz, D 2 O) δ2.91(s, 1H), 3.53(dd, J=9.9, 8.1Hz, 1H), 3.64-3.7...
Embodiment 3
[0103] Embodiment 3 contains the synthesis of triazole glycoside
[0104] 3.1 General method for synthesizing triazole-containing glycosides
[0105] Alkyne / azide-containing glycosides (0.5 mmol), organic azide / alkyne compounds (0.6 mmol), copper sulfate (9 mg, 0.05 mmol) sodium ascorbate (20 mg, 0.1 mmol) or copper powder ( 200 mg) was added to tert-butanol / water (1:1, 4.0 ml). Stir at room temperature for 4 to 24 hours, and obtain triazole-containing glycosides by column chromatography (eluent ethyl acetate / methanol / water=12~8 / 1 / 0.2).
[0106] 3.2 Spectral data containing triazole glycosides
[0107] 11:
[0108] Colorless syrup; [α] 25 D -29.4 (c2.30, CH 3 OH); 1 H NMR (300MHz, CD 3 OD) δ3.15(t, J=7.8Hz, 1H), 3.23-3.28(m, 4H), 3.62(dd, J=11.7, 4.8Hz, 1H), 3.83(d, J=11.7, 1H), 4.32(d, J=8.1Hz, 1H), 4.72(d, J=12.3Hz, 1H), 4.92(d, J=12.3Hz, 1H), 7.26-7.37(m, 5H), 7.97(s, 1H ); 13 C NMR (75MHz, CD 3 OD) δ54.9, 62.7, 63.0, 71.6, 75.0, 77.8, 78.0, 103.6, 125.3, 129.1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com