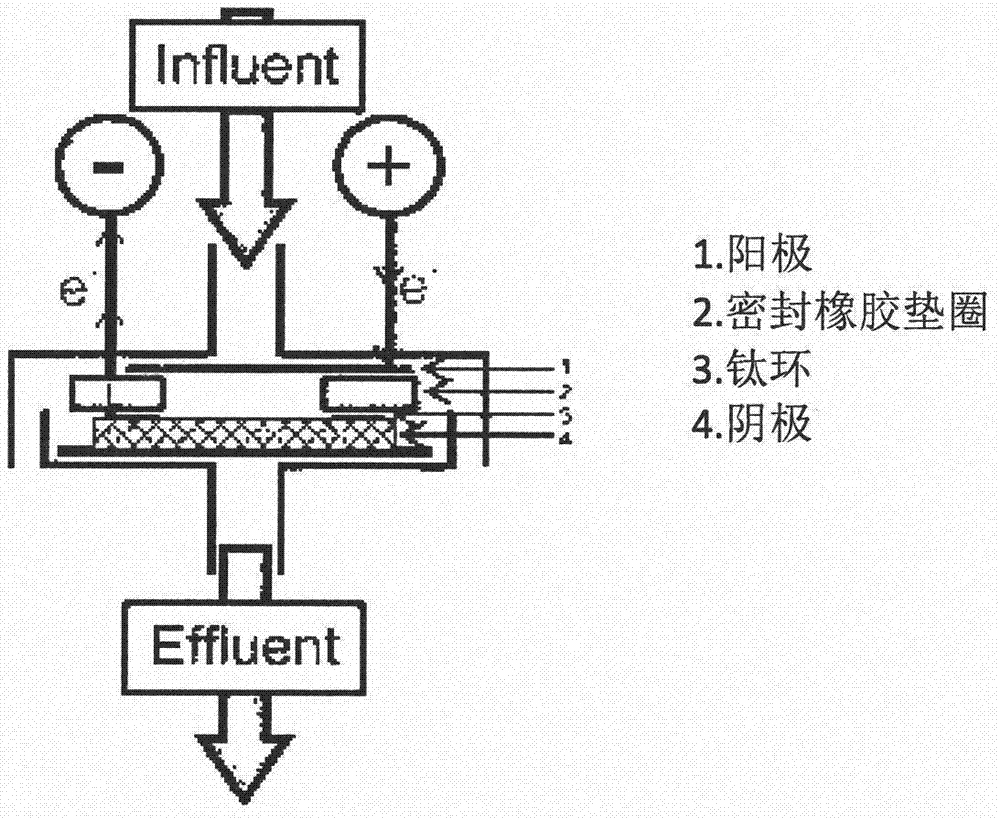
Method for removing perchlorate from water body through electrochemical reduction
A perchlorate and electrochemical technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., to achieve the effect of improving mass transfer efficiency, increasing reduction speed, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 1 g of carbon nanotubes into a three-necked flask, add 500 mL of concentrated sulfuric acid, stir magnetically, reflux at 70° C. for 12 h, and cool naturally to room temperature. The acidified solution of carbon nanotubes was vacuum filtered with a PTFE filter membrane with a pore size of 5 μm, and rinsed with a large amount of distilled water until the pH of the filtrate was neutral, and the rinsed carbon nanotubes were dried in an oven at 60° C.
[0024] Weigh 15 mg of carbon nanotubes treated with nitric acid, add 30 mL of distilled water, and disperse by ultrasonic. After the carbon nanotube mixture was naturally cooled to room temperature, 50 mg of SnCl was added 2 2H 2 O, add distilled water to 100mL, magnetically stir for 1h, add 100mgNaBH 4 , continue magnetic stirring for 2h, then vacuum filter the mixture onto a PTFE membrane, wash with a large amount of distilled water, and prepare a Sn@CNTs membrane electrode.
[0025] Add 30 mL of dimethyl sulfoxid...
Embodiment 2
[0031] The equipment used in the experiment and the experimental process are the same as in Example 1. The set voltage is 3V, and the Sn@CNTs film electrode is used as the cathode. The synthesis method is as in Example 1. When synthesizing composite materials, the mass of carbon nanotubes is 15mg. The difference is that SnCl 2 2H 2 The quality of O is different.
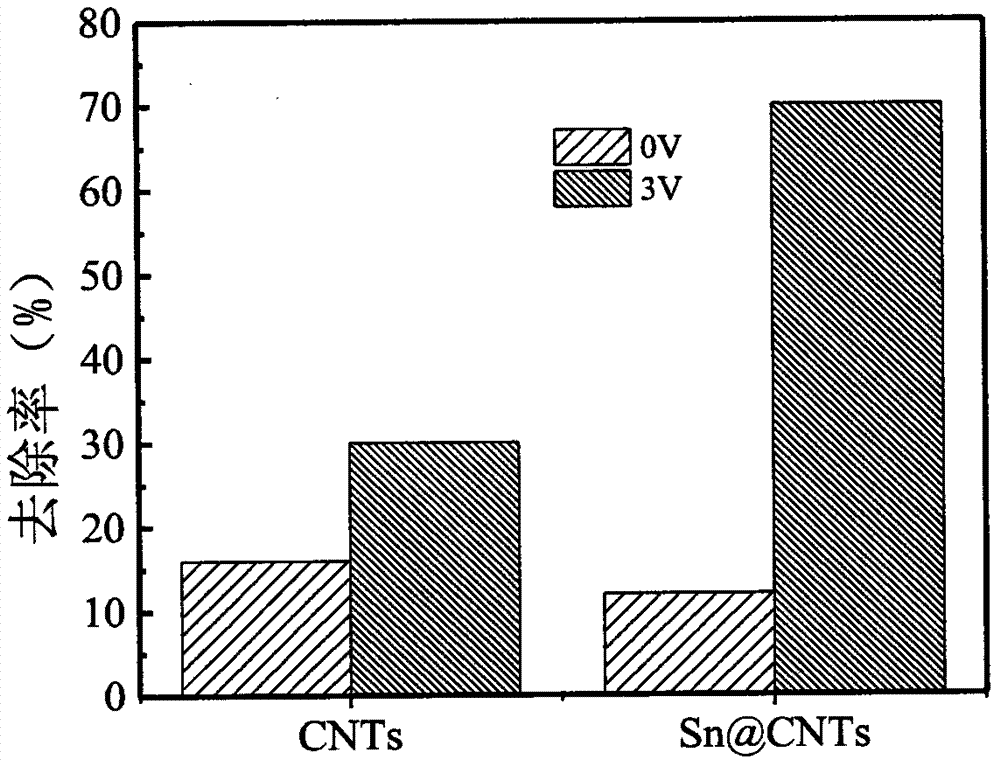
[0032] (1) SnCl 2 2H 2 The mass of O is 20 mg synthetic Sn@CNTs membrane electrode, and the removal rate of perchlorate is 28% after running for one hour, which is equivalent to that of carbon nanotube membrane electrode.
[0033] (2) SnCl 2 2H 2 The quality of O is 50 mg synthesized Sn@CNTs membrane electrode, running for one hour, the removal rate of perchlorate is 70%, which is 133% higher than that of carbon nanotube electrode.
[0034] (3) SnCl 2 2H 2 The quality of O is 100mg synthesized Sn@CNTs membrane electrode, running for one hour, the removal rate of perchlorate is 45%, which is 50% higher than tha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com