Alkyl phosphonate preparing method
A technology of hydrocarbyl phosphonate and hydrocarbyl phosphate, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of limited reaction substrates, large steric hindrance, carbon-halogen bond Low polarizability and other issues, achieve high reaction efficiency and conversion rate, and expand the effect of selection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
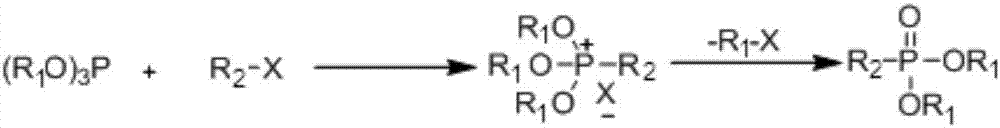
[0024] In order to solve this problem, the present invention provides a kind of preparation method of hydrocarbyl phosphonate, it comprises the following steps: compound A and compound B are carried out Arbuzov reaction in continuous reaction equipment, and in reaction process, react The product is continuously discharged from the continuous reaction equipment to obtain hydrocarbyl phosphonate; wherein compound A is shown in structure I, and compound B is shown in structure II:
[0025]
[0026] Wherein, in structure I, X is a halogen atom, R 1 Is an alkyl or substituted alkyl, wherein the substituent of the substituted alkyl is aryl, ester, epoxy, alkenyl or acyl; in structure II, R is methyl, ethyl or phenyl; and during the reaction The reaction temperature is T1, the boiling point of compound A and compound B with the lower boiling point under standard atmospheric pressure is T2, T1 is 10-40°C higher than T2, and the reaction pressure during the reaction is 0.5-2.0MPa. ...
Embodiment 1
[0041]
[0042] Heat the dry and clean 500mL coil to 170°C, take 1000g (0.7eq.8.16mol) of compound 1 (boiling point at standard atmospheric pressure 142-145°C) into the feeding bottle A, add 4000g of toluene to dilute, and take the phosphorous acid 1937g (1.0eq.11.66mol) of triethyl ester (boiling point at standard atmospheric pressure is 156.6°C) is placed in the feeding bottle B, and 3063g of toluene is added for dilution. solution): 16.7 g / min, pump B (triethyl phosphite in toluene): 16.7 g / min. The residence time is 15min, and the reaction pressure is 0.5-2.0Mpa. The discharge port is directly connected to the thin film evaporation device, and the control pressure is 4~10×10 2 Pa, temperature 105-115 DEG C, finally obtain product 2 (boiling point 129-131 DEG C, 800 Pa) 1701g, yield 93%.
[0043] 1 H NMR (400MHz, CDCl 3 ):δ4.14–4.21(m,4H),3.86–4.01(m,2H),3.00(d,2H),1.35(t,J=6.8Hz,3H),1.22(t,J=6.8Hz, 3H).
Embodiment 2
[0045]
[0046] Heat the dry and clean 500mL coil to 155°C, take 1000g of compound 1 (0.7eq.8.16mol, the boiling point at standard atmospheric pressure is 142-145°C) into the bottle A, add 4000g of toluene to dilute; take phosphorous acid 1937g (1.0eq.11.66mol) of triethyl ester was placed in the feeding bottle B, and 3063g of toluene was added to dilute it. After the temperature of the coil was stable, the feeding was started. Pump A (toluene solution of compound 1): 16.7g / min, pump B (toluene solution of triethyl phosphite): 16.7 g / min. The residence time is 15min, and the reaction pressure is 2Mpa. The discharge port is directly connected to the thin film evaporation device, and the control pressure is 4~10×10 2 Pa, the temperature is 140-145° C., and finally 1683 g of product 2 is obtained with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com