An analytical method for determining stereoisomers and intermediates in solifenacin succinate
A technology for solifenacin succinate and stereoisomers, which is applied in the field of analysis of stereoisomers and intermediates in solifenacin succinate, and can solve problems such as detection of two intermediate components not included, To achieve the effect of improving the detection method, reducing the detection cost and ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
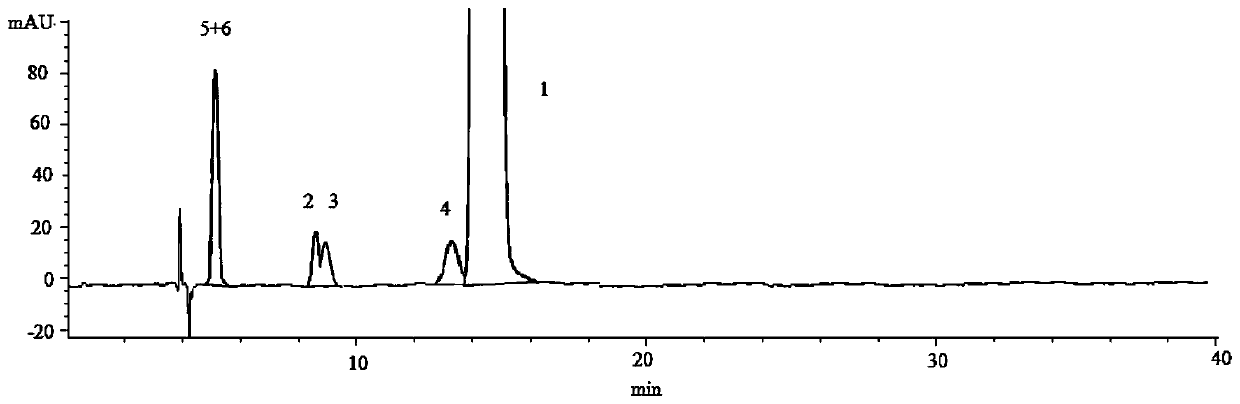
[0051] Determination of Stereoisomers and Intermediates in Solifenacin Succinate
[0052] (1) Instrument and chromatographic conditions
[0053] High performance liquid chromatography: Agilent 1260 high performance liquid chromatography system and workstation.
[0054] Chromatographic column: amylose-tris(3,5-xylylcarbamate) bonded silica gel chromatographic column (CHIRALPAKIA, 4.6 mm×250 mm, 5 μm).
[0055] The mobile phase is n-hexane-absolute ethanol-isopropanol-diethylamine (80:10:10:0.1) (volume ratio), the set flow rate is 1.0mL / min, the detection wavelength is 220nm, and the column temperature is 25°C.
[0056] (2) Experimental steps
[0057] Take solifenacin succinate sample and appropriate amount of impurity B, C, D, E, F reference substance respectively, dissolve and dilute with mobile phase to make about 1 mg of solifenacin succinate and about 5 μg of each impurity in 1 mL The mixed solution was used as the test solution.
[0058] (3) Measurement method and res...
Embodiment 2
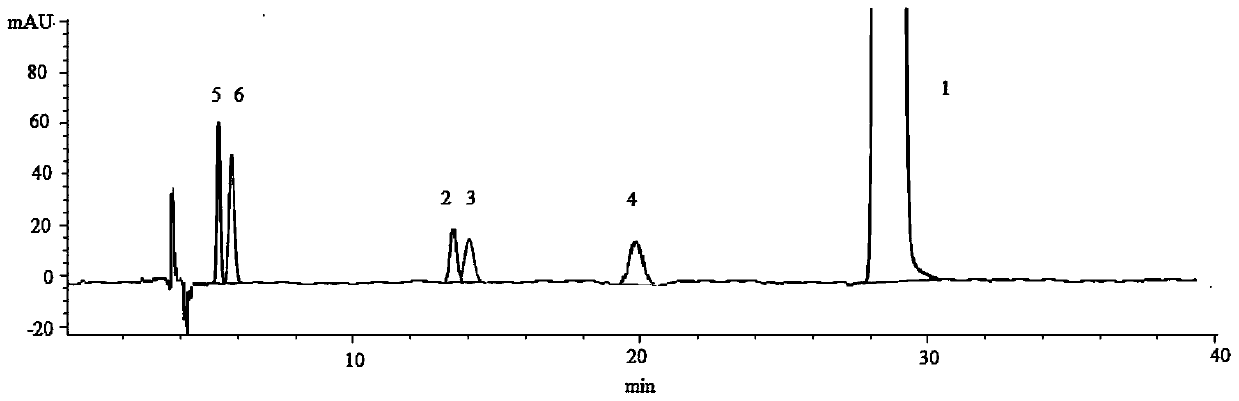
[0061] Determination of Stereoisomers and Intermediates in Solifenacin Succinate
[0062] (1) Instrument and chromatographic conditions
[0063]High performance liquid chromatography: Agilent 1260 high performance liquid chromatography system and workstation.
[0064] Chromatographic column: amylose-tris(3,5-xylylcarbamate) bonded silica gel chromatographic column (CHIRALPAKIA, 4.6 mm×250 mm, 5 μm).
[0065] The mobile phase is n-hexane-absolute ethanol-isopropanol-diethylamine (50:25:25:0.1) (volume ratio), the set flow rate is 1.0mL / min, the detection wavelength is 220nm, and the column temperature is 25°C.
[0066] (2) Experimental steps
[0067] Take solifenacin succinate sample and appropriate amount of impurity B, C, D, E, F reference substance respectively, dissolve and dilute with mobile phase to make about 1 mg of solifenacin succinate and about 5 μg of each impurity in 1 mL The mixed solution was used as the test solution.
[0068] (3) Measurement method and resu...
Embodiment 3
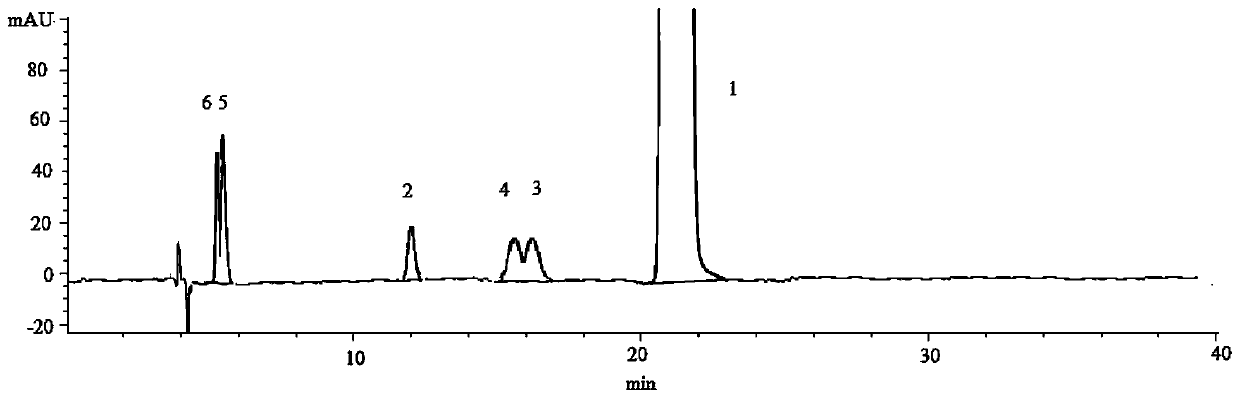
[0071] Determination of Stereoisomers and Intermediates in Solifenacin Succinate
[0072] (1) Instrument and chromatographic conditions
[0073] High performance liquid chromatography: Agilent 1260 high performance liquid chromatography system and workstation.
[0074] Chromatographic column: amylose-tris(3,5-xylylcarbamate) bonded silica gel chromatographic column (CHIRALPAKIA, 4.6 mm×250 mm, 5 μm).
[0075] The mobile phase is n-hexane-absolute ethanol-isopropanol-diethylamine (65:15:20:0.1) (volume ratio), the set flow rate is 1.0mL / min, the detection wavelength is 220nm, and the column temperature is 20°C.
[0076] (2) Experimental steps
[0077] Take solifenacin succinate sample and appropriate amount of impurity B, C, D, E, F reference substance respectively, dissolve and dilute with mobile phase to make about 1 mg of solifenacin succinate and about 5 μg of each impurity in 1 mL The mixed solution was used as the test solution.
[0078] (3) Measurement method and res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com