Polypeptide for inhibiting tumor cell diffusion transfer and preparation method and application thereof
A tumor cell and polypeptide chain technology, applied in the field of biopharmaceuticals, can solve the problem of few polypeptide reports, and achieve the effects of no toxic side effects, small molecular weight, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
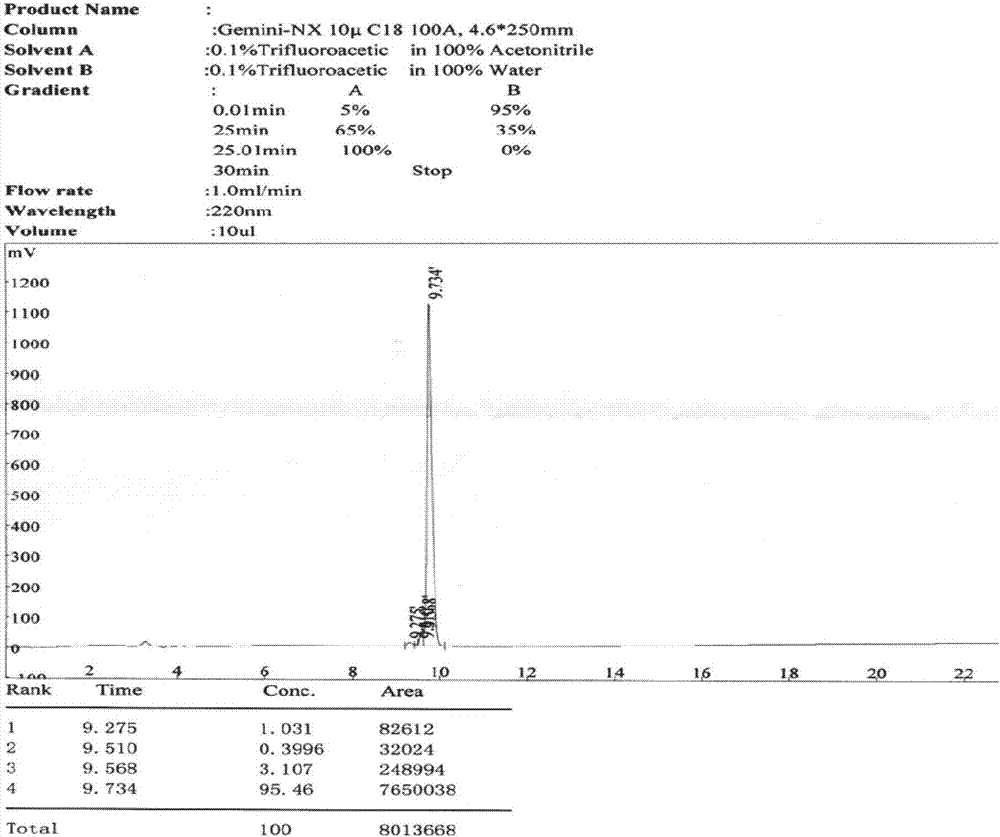
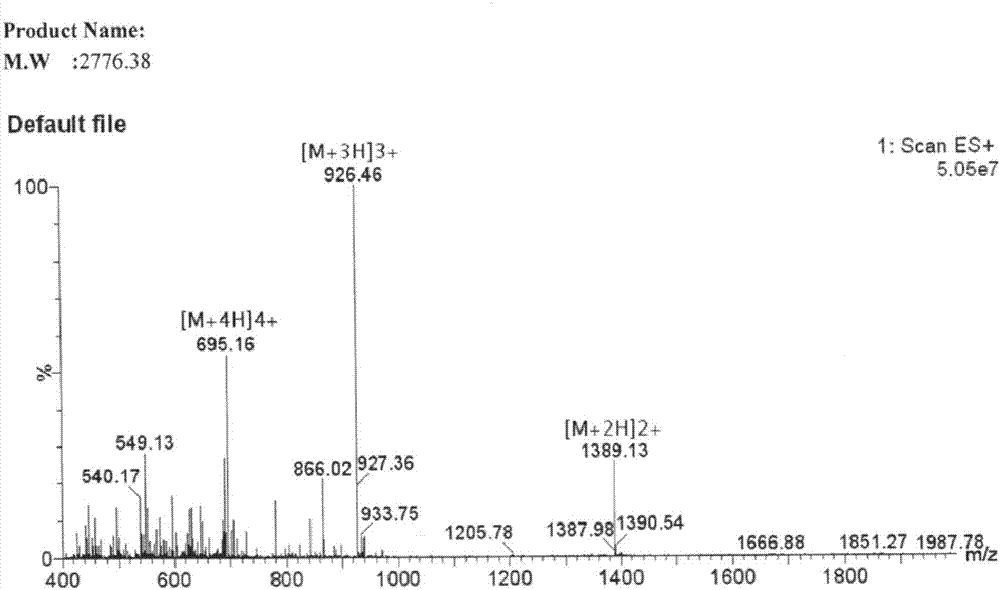
[0029] Embodiment 1 prepares polypeptide of the present invention
[0030] The following 2-CL resins for preparing polypeptides, Fmoc protected amino acids, condensation reagents, and cleavage reagents were all purchased from domestic biochemical reagent companies.
[0031] 1.1 The synthesis of polypeptide resin of the present invention
[0032] Polypeptide resin of the present invention is:
[0033] Lys(Boc)-Lys(Boc)-Lys(Boc)-Lys(Boc)-Asp(otbu)-Lys(Boc)-Ser(tbu)-Ser(tbu)-Phe-Ile-Ser(tbu)-Val -Leu-Gln(trt)-Thr(tbu)-Ser(tbu)-Ser(tbu)-Ser(tbu)-Ser(tbu)-Leu-Arg(Pbf)-Met-Gly-Ala-Tyr(tbu) - 2-CL resin.
[0034] Using 2-CL resin as the initial carrier, the polypeptide resin of the present invention was prepared by de-Fmoc protection and coupling reaction, followed by coupling with the protected amino acids shown in Table 1. The protected amino acids used in this embodiment correspond to the 1st to 25th amino acids from the resin as shown in the following table:
[0035] Table 1...
Embodiment 2
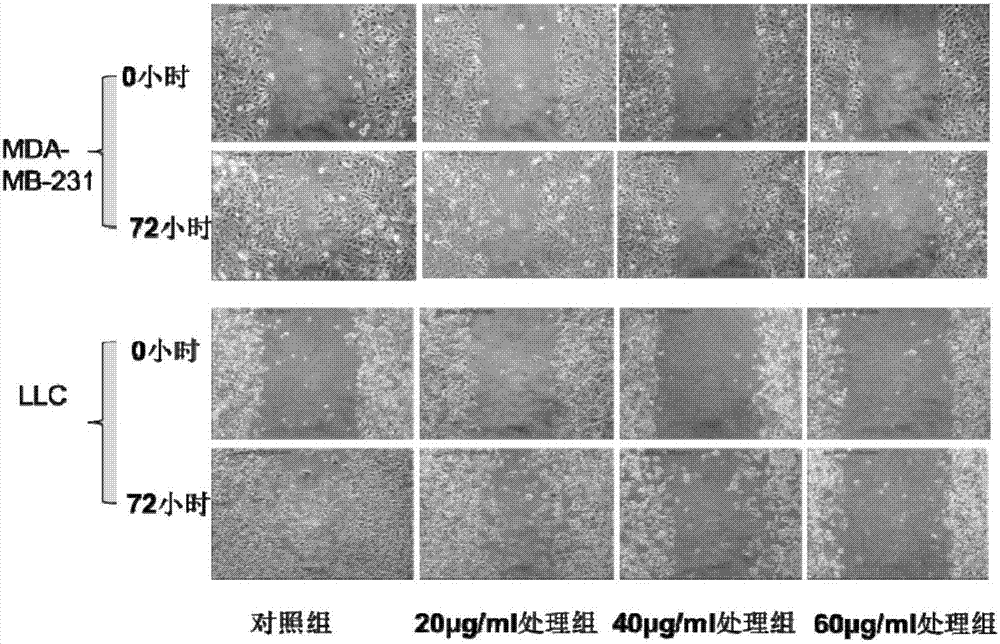
[0054] Example 2 Detection of Polypeptide of the Present Invention Inhibiting Tumor Cell Migration in Vitro
[0055] The polypeptide of the present invention is prepared by the method of Example 1, and the serum-free medium is used to prepare 1 mg / mL, stored in a refrigerator at 4 degrees, and stored in a refrigerator at -20 degrees Celsius for a long time. Human colon cancer sw620 cell line, LLC tumor cells and breast cancer MDA-MB-231 cell line were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. The medium used in sequence is 1640RPMI, DMEM.
[0056] 2.1 Use the scratch method to detect the effect of the polypeptide of the present invention on the migration of different tumor cells in vitro
[0057] Human breast cancer MDA-MB-231 cells in logarithmic growth phase and mouse lung cancer LLC tumor cells were inoculated in 12-well plates, and cultured in their respective culture media. Scratching was performed when the cells were 90% confluent. After scratchi...
Embodiment 3
[0060] Example 3 Effect of the polypeptide of the present invention on lung metastasis of mouse lung cancer LLC tumor cells in vivo
[0061] Congeneric Kunming mice of 25-35 g were divided into control group 1, control group 2 and treatment group, with 9 mice in each group. Control group 1 was injected with 0.1 mL of mouse lung cancer LLC tumor cells through the tail vein, about 1.5×10 6 cells; control group 2 was injected with 0.1 mL of mouse lung cancer LLC tumor cells through the tail vein, about 1.5×10 6 cells (containing low water-soluble natural polypeptide 50 μg / mL, amino acid sequence is: DKSSFISVLQTSSSSLRMGAY, has applied for a patent.); the treatment group was injected with 0.1 mL of mouse lung cancer LLC tumor cells through the tail vein, about 1.5 × 10 6 cells (containing 50 μg / mL of the polypeptide of the present invention). A mouse model of lung metastasis of lung cancer LLC tumor cells was established. The mice were killed four weeks after the injection, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com