Green silicate long afterglow luminescent material and preparation method thereof
A technology for long-lasting luminescent and luminescent materials, which is applied in the preparation of the luminescent materials, long-lasting luminescent materials, and silicate long-lasting luminescent materials. It can solve the problems of improving the intensity and duration of afterglow luminescence, and achieve good chemical stability. and thermal stability, the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of the green silicate long afterglow luminescent material is specifically carried out according to the following steps:
[0021] Step 1: To contain potassium ion K + , Barium ion Ba 2+ , silicon ion Si 4+ , rare earth ion Eu 2+ and R III The compound is the raw material, according to the chemical expression K 2 Ba 7-x-y Si 16 o 40 : xEu 2+ , yR III The stoichiometric ratio of each element in the raw material is weighed, and in the chemical expression, R III is the rare earth ion Tb 3+ 、Ce 3+ 、Dy 3+ 、Tm 3+ 、Nd 3+ 、Gd 3+ , Y 3+ 、Er 3+ , La 3+ 、Pr 3+ 、Sm 3+ , Yb 3+ 、Lu 3+ 、Ho 3+ one or both of the
[0022] Grind and mix the obtained raw material powders evenly;
[0023] Step 2: Put the raw material powder obtained in Step 1 in an environment with a temperature of 1050°C to 1250°C, and calcinate in a reducing atmosphere for 3 to 6 hours;
[0024] Three kinds of gases can be used in the reducing atmosphere: the first is ammoni...
Embodiment 1
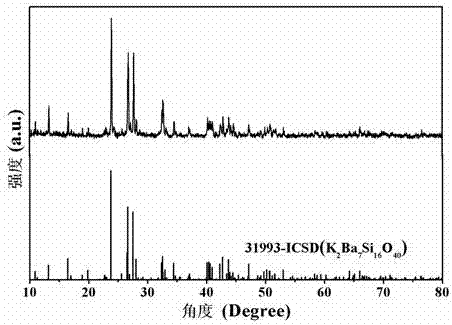
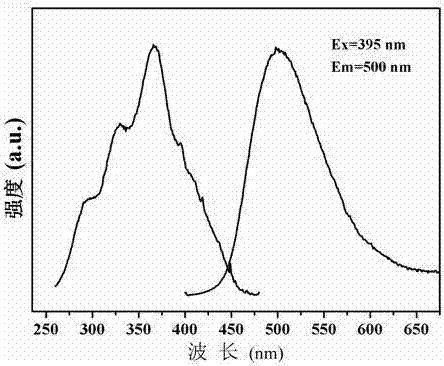
[0029] press K 2 Ba 6.98 Si 16 o 40 : 0.01Eu 2+ , 0.01Ho 3+ Stoichiometric ratio shown in molecular formula, weigh 0.0691g K 2 CO 3 , 0.6887g BaCO 3 , 0.4807g SiO 2 , 0.0009g Eu 2 o 3 and 0.0009g Ho 2 o 3 As a raw material, the weighed raw materials are ground and mixed evenly, put into an alumina crucible, placed in an environment with a temperature of 1250 ° C, and calcined for 3 hours in a reducing atmosphere. The reducing atmosphere is composed of 95% nitrogen and 5% nitrogen by volume. Composition of hydrogen gas, the calcined raw material powder is cooled to room temperature with the furnace to obtain a calcined product; after grinding, a luminescent material is obtained. figure 1 Shown is the XRD pattern of the luminescent material, indicating that the phase of the luminescent material is K 2 Ba 7 Si 16 o 40 . Excitation and emission spectra of the luminescent material, such as figure 2 As shown, the figure shows that the emission spectrum of the long...
Embodiment 2
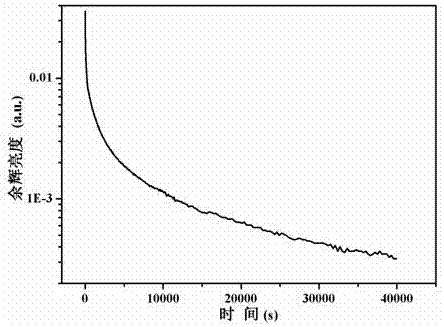
[0031] press K 2 Ba 6.99 Si 16 o 40 : 0.005Eu 2+ , 0.005Nd 3+ The stoichiometric ratio shown in the molecular formula weighs 0.0691gK 2 CO 3 , 0.6897g BaCO 3 , 0.4807g SiO 2 , 0.0004g Eu 2 o 3 and 0.0005g Nd 2 o 3 As a raw material, the weighed raw materials are ground and mixed evenly, put into an alumina crucible, placed in an environment with a temperature of 1150 ° C, and calcined for 5 hours under a reducing atmosphere. The reducing atmosphere is composed of 75% nitrogen and 25% by volume. Composition of hydrogen gas, the calcined raw material powder is cooled to room temperature with the furnace to obtain a calcined product; after grinding, a green silicate long-lasting luminescent material is obtained. Figure 5 0.0010g sample K 2 Ba 6.99 Si 16 o 40 : 0.005Eu 2+ , 0.005Nd 3+ The pyrolysis spectrum measured after the light source with a wavelength of 254nm and a light source with a wavelength of 365nm irradiated for 2 minutes at the same time. It can b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com