Method for efficient separation and recycling of epoxy isomer in telapristone synthesis
A telapristone and isomer technology, applied in the field of efficiently obtaining 5,10-α epoxy isomers in the synthesis process of telapristone, can solve the problems of coma, volatile, flammable and explosive, etc. , to achieve the effects of improving utilization efficiency, convenient operation, excellent yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
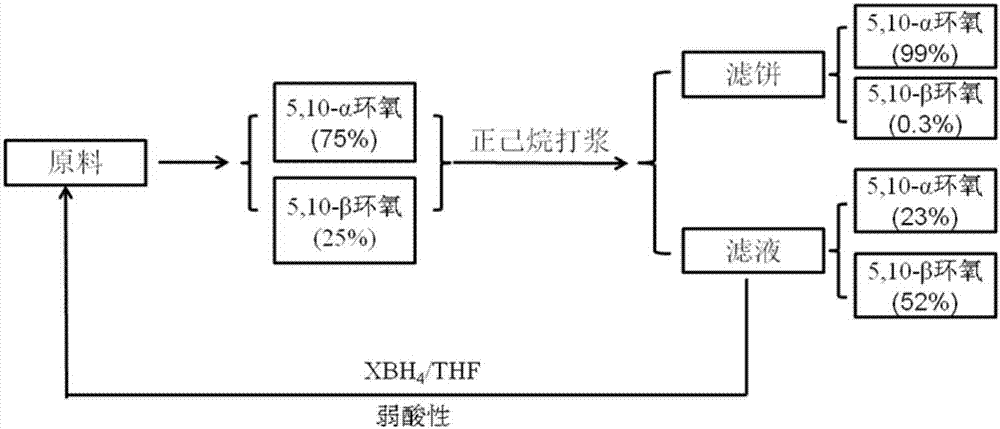
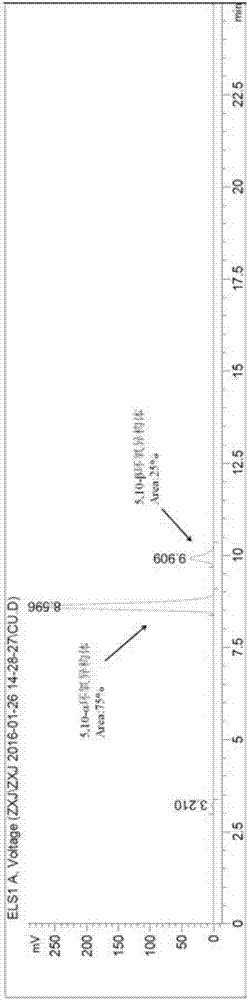
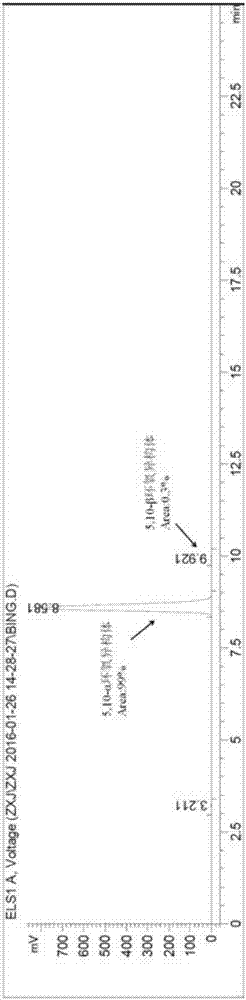
[0047] Example 1: Synthesis and separation of epoxy isomer 5,10-α / β
[0048] In a 1000mL three-neck flask equipped with a thermometer, a constant pressure titration funnel, and a glass stopper, add 17β-cyano-17α-trimethylsilyloxy-13β-methylsterane-5,9-diene-3, 3-(Ethylenedioxy) (52.2g, 0.117mol), add 300mL of dichloromethane, stir vigorously for 20min to dissolve the material completely, add disodium hydrogen phosphate (18.1g, 0.127mol), hexafluoroacetone (2.26 mL, 0.0252mol), it was placed in an ice-salt bath and continued to stir for 20 min, and slowly added 30% H 2 o 2 (73.0mL, 0.643mol), after the reaction is stable without exothermic heat, it can be placed at room temperature and continue to stir vigorously. After 24 hours of reaction, TLC detects that the reaction is complete. Slowly add 50mL saturated sodium thiosulfate, stir for 1h, let stand to separate the layers, separate the organic phase, and use saturated Na 2 SO 4 , saturated NaHCO 3 , saturated NaCl to was...
Embodiment 2
[0050] Embodiment 2: the reduction recycling of mother liquor
[0051] Take 20 g of the above-mentioned concentrated mother liquor and place it in a 100 mL single-necked bottle, dissolve it in 100 mL THF, stir and dissolve under an ice bath, add a reducing agent (70 mmol), maintain the system temperature and stir for 2 h, after TLC detects that the reaction is complete, add 2% paraffin Aqueous toluenesulfonic acid solution (20mL), stirred for 30min, then added 200mL dichloromethane and allowed to stand for stratification, and the organic phase was sequentially washed with 20mL saturated NaHCO 3 , washed the organic phase with 20 mL saturated NaCl, anhydrous NaCl 2 SO 4 Dry, filter, and concentrate under reduced pressure to obtain 12.5 g of 17β-cyano-17α-trimethylsilyloxy-13β-methylsterane-5,9-diene-3,3-(ethylenedioxy) , the yield was 65%. 1 H NMR (400MHz, CDCl 3 )δ5.62(t,J=4Hz,1H),3.98(s,4H),2.57-0.79(m,18H),0.89(s,3H),0.23(s,9H). 13 C NMR (100MHz, CDCl 3 ): δ135.88, 130...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com