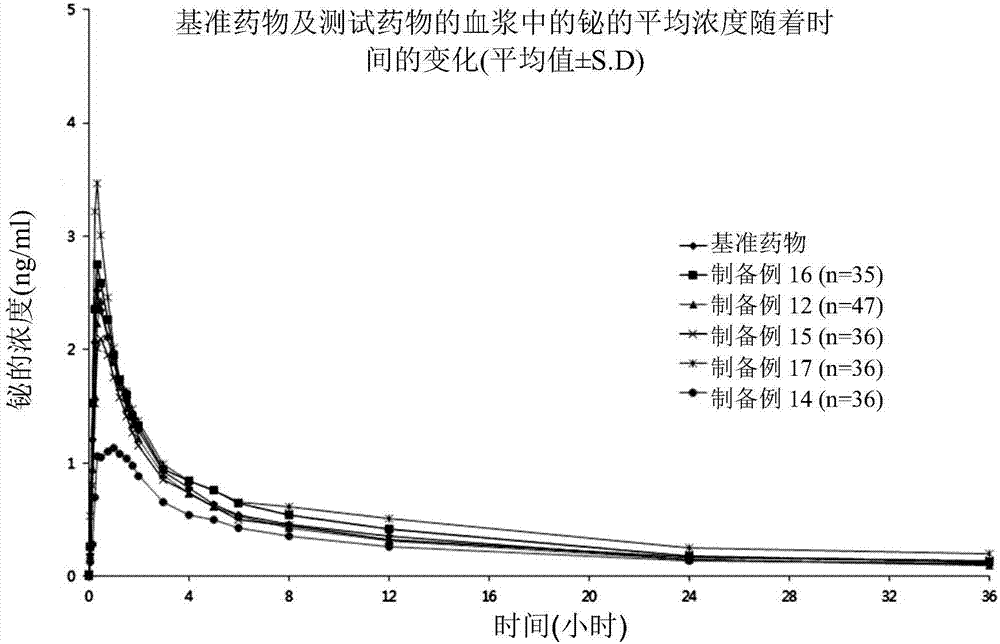
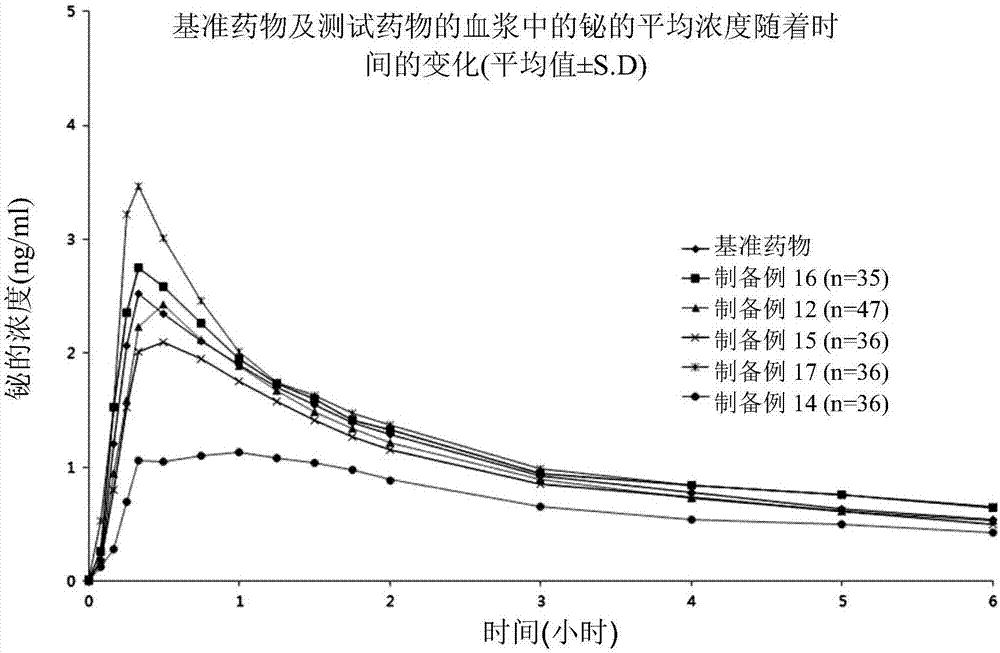
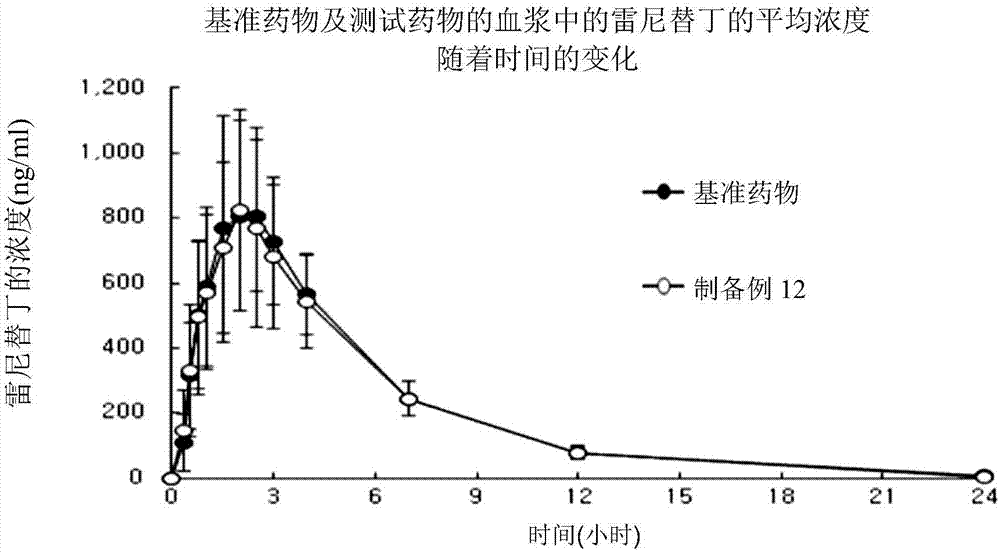
A pharmaceutical composition for treating gastrointestinal diseases
A technology for gastrointestinal diseases and compositions, which is applied in the field of pharmaceutical compositions for the treatment of gastrointestinal diseases, and can solve problems such as reducing the stability of ranitidine, uncomfortable swallowing by patients, and complicated preparation processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: SK Adjustment of Particle Size of Lafite and Bismuth Subcitrate
[0080] Ranitidine, sclaffil, and bismuth subcitrate having the raw material particle sizes before micronization in Table 1 were used in the experiment.
[0081] 【Table 1】
[0082] Raw material particle size before micronization
[0083] Particle size distribution
Scraffi
bismuth subcitrate
d(10)
2.59μm
21.172μm
18.565μm
d(50)
8.55μm
75.135μm
72.380μm
d(90)
20.74μm
176.62μm
195.559μm
average
10.54μm
89.154μm
95.563μm
[0084] The raw material particle diameter was measured according to a wet method using a particle diameter measuring device (Malvern Mastersizer 2000 / Hydro S) under the following conditions.
[0085]
[0086] Device: laser diffraction particle size distribution measurement device Malvern Mastersizer 2000 / Hydro S (Malvern)
[0087] Wet unit: Hydro 2000S
[0088] Sam...
experiment example 1
[0112] Experimental Example 1: Adjustment of the particle size of scrafil
[0113] In order to verify the change of the dissolution rate of scrafil according to the particle size adjustment of sclafil, as shown in Table 5, use and prepare raw materials with particle size combinations according to the composition of table 4, wherein only the particle size of sclafil is changed, At the same time the particle sizes of ranitidine and bismuth subcitrate were fixed, and then the experiment was performed.
[0114] 【table 5】
[0115] Combination of Particle Sizes of Ranitidine, Sclafin and Bismuth Subcitrate
[0116]
[0117] Except that the raw materials have different particle sizes, according to the same composition as shown in Table 4, use pure water (or a mixed solvent of pure water and ethanol) to prepare dry granules or wet granules according to the usual method, and dry at 50 ° C. And then pass through a 18-mesh sieve. Then, the prepared granules were compressed and co...
experiment example 2
[0136] Experimental Example 2: Adjustment of Particle Size of Bismuth Subcitrate
[0137] Based on the results of Experimental Example 1, it was defined that the raw materials having the particle diameters represented by scrafil 4 to sclafil 6 in Table 2 are preferably used as sclafil. Therefore, the particle size of sclafil was fixed to the particle size of "sclafil 6", and then the particle size of bismuth subcitrate was adjusted to evaluate the stability of the tablet according to the particle size of bismuth.
[0138] Raw materials with particle size combinations in Table 7 were used and formulated according to the composition in Table 4, and then experiments were performed.
[0139] 【Table 7】
[0140] Combination of Particle Sizes of Ranitidine, Sclafin and Bismuth Subcitrate
[0141]
[0142] Except that the raw materials have different particle sizes, according to the same composition as shown in Table 4, ranitidine, sclaffil and hypocitrate were prepared by usin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com