Preparation method of super-hydrophobic powder based on flower-shaped iron-containing manganese dioxide and capable of being simultaneously used for emulsion separation and dye adsorption
A manganese dioxide and emulsion separation technology, which is applied in chemical instruments and methods, iron compounds, alkali metal oxides/hydroxides, etc., can solve the problems of complicated preparation steps, high production costs, and poor practicability of superhydrophobic materials. Achieve the effect of low cost, easy to obtain raw materials and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
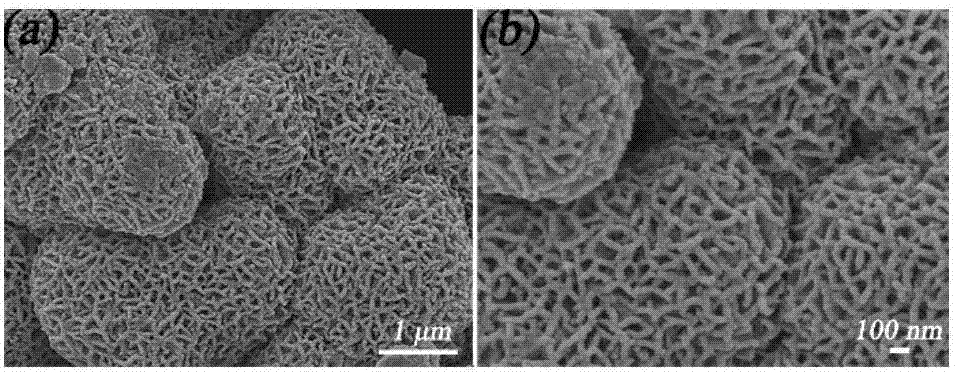
[0026] (1) Preparation of flower-shaped iron-containing manganese dioxide: Dissolve 3.56 g of manganese chloride and 4.56 g of ammonium persulfate in 160 ml of deionized water, and form a transparent solution under magnetic stirring. After that, dissolve 1.11 g of iron sulfite into In 40ml of deionized water, add ferric sulfite solution to solution one, transfer the mixed solution to an airtight container and place it at 80°C for 6h reaction, cool down to room temperature after the reaction, filter with ethanol and deionized water, respectively After washing three times, and then drying in a vacuum environment at 40° C. to obtain brown-black powder, the preparation of flower-like iron-containing manganese dioxide is completed.
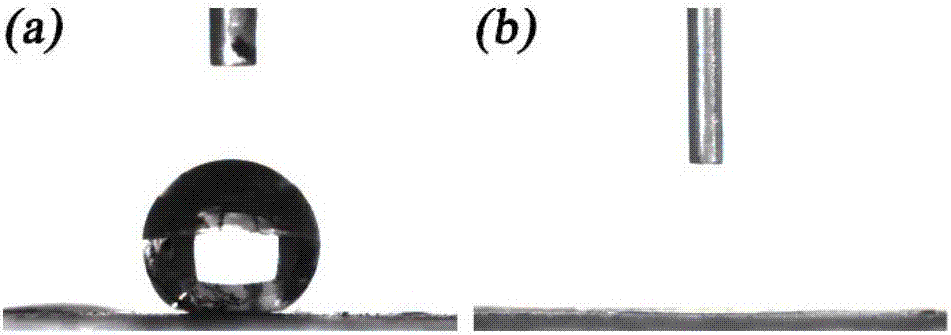
[0027] (2) Modification of low surface energy: The prepared powder was added to 50 mL of a solution mixed with 0.1 M stearic acid and ethanol, stirred magnetically for 12 h at room temperature, filtered and dried in vacuo to obtain a superhydrophobic po...
Embodiment 2
[0032] (1) Preparation of flower-shaped iron-containing manganese dioxide: 1.78g of manganese chloride and 2.28g of ammonium persulfate were dissolved in 80mL of deionized water, and a transparent solution was formed under magnetic stirring. After that, 0.56g of iron sulfite was dissolved in In 40 mL of deionized water, add the ferric sulfite solution to solution one, transfer the mixed solution to a closed container and place it at 80 °C for 6 hours of reaction. After washing three times, and then drying in a vacuum environment at 40° C. to obtain brown-black powder, the preparation of flower-like iron-containing manganese dioxide is completed.
[0033] (2) Modification of low surface energy: The prepared powder was added to 50 mL of a solution mixed with 0.1 M stearic acid and ethanol, stirred magnetically for 12 h at room temperature, filtered and dried in vacuo to obtain a superhydrophobic powder.
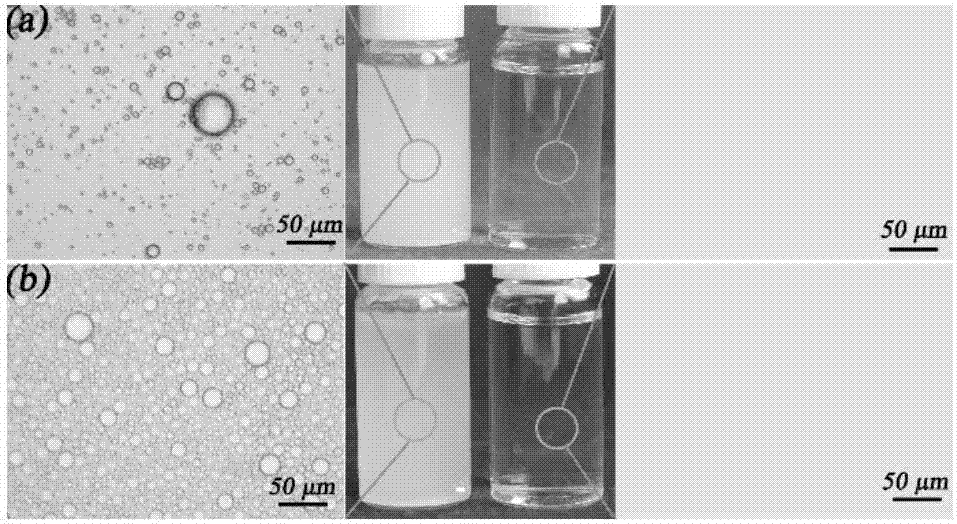
[0034] (3) Separation of emulsion: 0.05g of superhydrophobic powder was ad...
Embodiment 3
[0038] (1) Preparation of flower-shaped iron-containing manganese dioxide: Dissolve 0.89 g of manganese chloride and 1.14 g of ammonium persulfate in 40 ml of deionized water to form a transparent solution one under magnetic stirring, and then dissolve 0.28 g of iron sulfite into In 40ml of deionized water, add ferric sulfite solution to solution one, transfer the mixed solution to an airtight container and place it at 80°C for 6h reaction, cool down to room temperature after the reaction, filter with ethanol and deionized water, respectively After washing three times, and then drying in a vacuum environment at 40° C. to obtain brown-black powder, the preparation of flower-like iron-containing manganese dioxide is completed.
[0039] (2) Modification of low surface energy: The prepared powder was added to 50 mL of a solution mixed with 0.1 M stearic acid and ethanol, stirred magnetically for 12 h at room temperature, filtered and dried in vacuo to obtain a superhydrophobic powd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Contact angle | aaaaa | aaaaa |
| Roll angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com