Preparation of monoclonal antibody of OTUD5 and application of OTUD5 in cancer therapy
A monoclonal antibody and breast cancer technology, applied in the field of medicine and biology, can solve problems that other OUT family research does not involve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Screening out OTUD5 in nude mice as a new gene associated with breast cancer metastasis
[0028] 1. In vivo screening model in nude mice
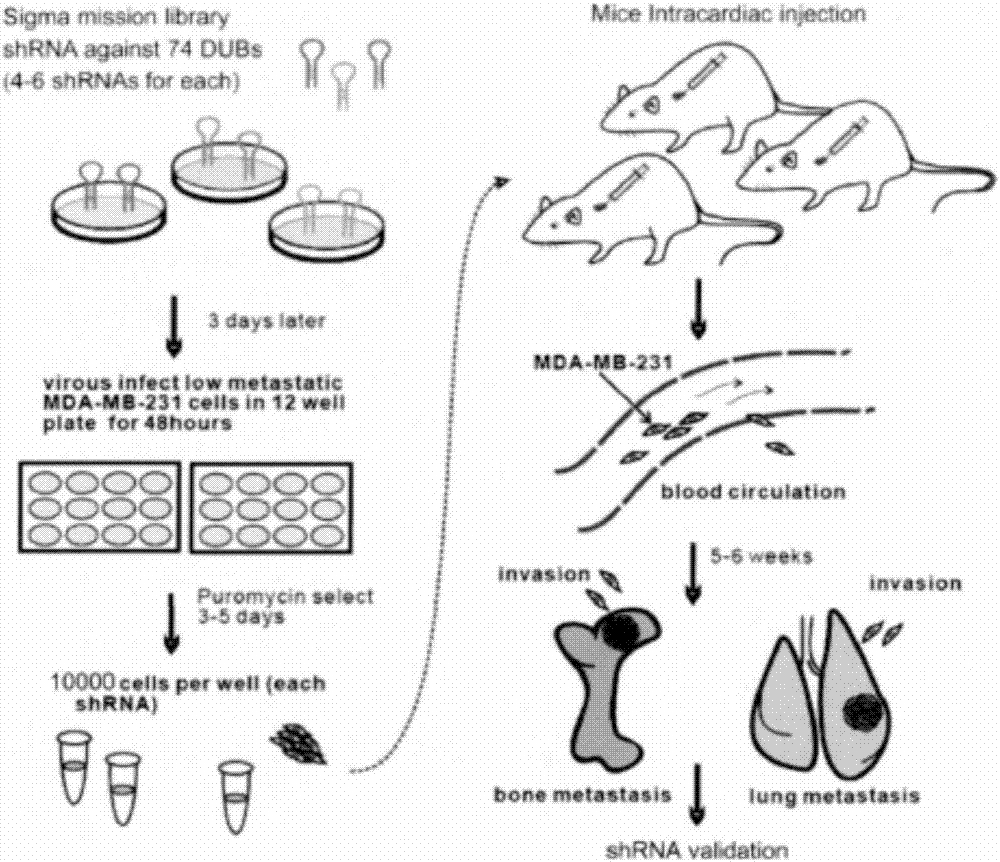
[0029] In this study, we designed an in vivo metastasis model using nude mice to screen deubiquitinating enzymes that can inhibit breast cancer metastasis, and applied it to MDA-MB-231 cells with epithelial-like traits and low metastatic ability. We used A shRNA library targeting 75 deubiquitinating enzymes (the library was purchased through Sigma mission library and Beijing Aurui Dongyuan Biotechnology Co., Ltd.), each deubiquitinating enzyme has 4-6 different shRNAs, of which at least Two have been identified as effective. First, we packaged and produced 371 different shRNA viruses using HEK293T cells, and infected them into a sufficient number of MDA-MB-231 cells in a 12-well plate. After 48 hours, we used Puromycin at a concentration of 2 μg / ml to obtain 371 stable cell lines after 3 days of selection. We then mixed ...
Embodiment 2
[0031] Example 2 Down-regulation of OTUD5 expression inhibits lung metastasis of breast cancer
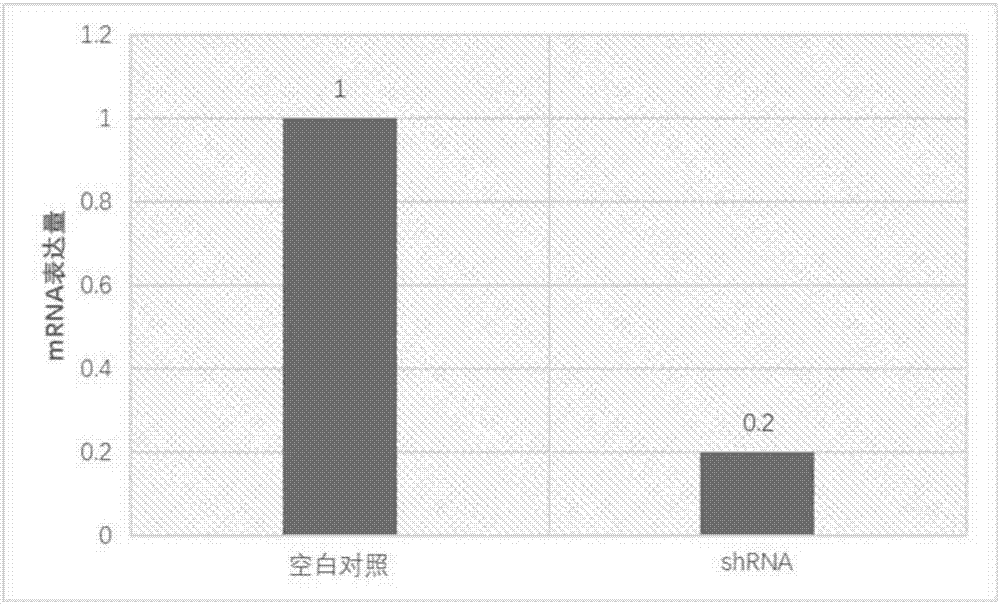
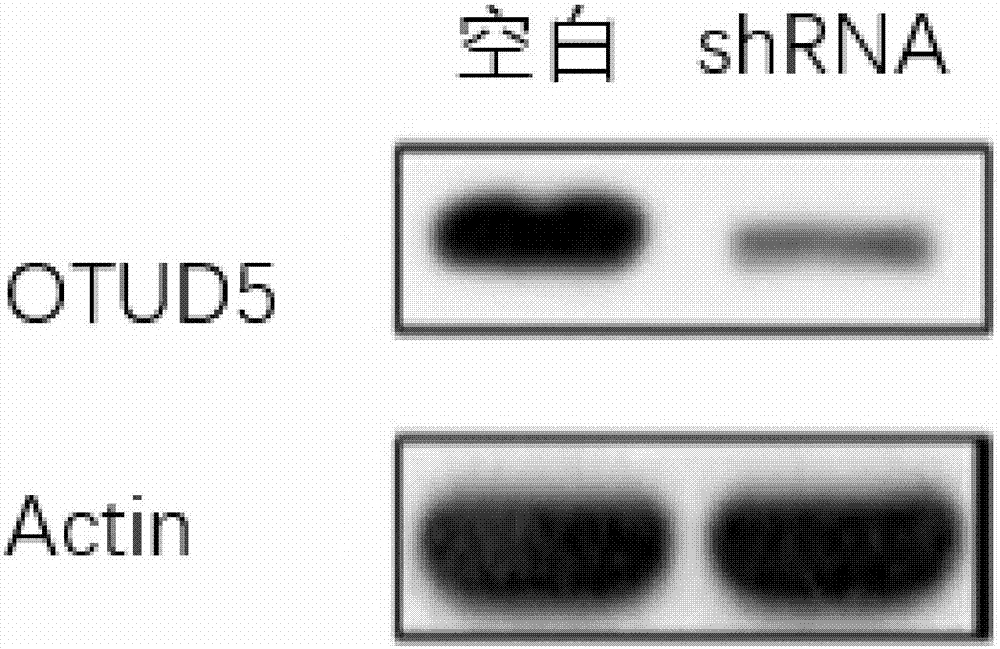
[0032] In order to verify the role of OTUD5 in tumor metastasis, we used shRNA interference technology (OTUD5 (Locus ID55593) Human shRNA, purchased from Beijing Aurui Dongyuan Biotechnology Co., Ltd.) to screen and obtain OTUD5 knockout MDA-MB-231 cells Department, from figure 2 It can be seen that the mRNA expression of OTUD5 was reduced by 80%, image 3 The amount of protein expression was calculated by graphing software, and the amount of protein expression was reduced by 79%. This shows that the gene knockout cells were constructed successfully.
[0033] After injecting this cell line into the hearts of nude mice, there were 30 mice in each group, and one group was the MDA-MB-231 cell line without knockout. After 8 weeks of reaction, the bone metastasis of the shRNA-introduced cell line was only 10%, while the bone metastasis of the cell line without shRNA reached 66.7%, w...
Embodiment 3
[0034] Preparation of embodiment 3 OTUD 5 monoclonal antibody
[0035] 1. Generation of CHO cells stably expressing OTUD 5 antigen
[0036] According to the NM_001136157 sequence, primers were designed to amplify the full-length human OTUD 5 cDNA, which was connected to the expression vector pcDNA3.1(+) to construct the eukaryotic expression vector pcDNA3.1(+)-OTUD 5.
[0037] (2) Transfection of CHO cells
[0038] Inoculate 3X10 in a 3.5cm tissue culture dish 5 CHO cells, when the cells were cultured to 90%-95% confluence, transfected: 10 μg of plasmid (pcDNA3.1(+)-OTUD 5) and 20 μl of Lipofectamine2000 Reagent (product of Invitrogen Company) were dissolved in 500 μl of serum-free DMEM medium respectively, and static at room temperature Set aside for 5 minutes, mix the above two liquids, and incubate at room temperature for 20 minutes to form the DNA-liposome complex, during which time, replace the serum-containing medium in the petri dish with 3ml of serum-free DMEM medium, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com