Preparation method of ruthenium-carbene catalyst
A carbene catalyst and halide technology, which is applied in the field of preparation of ruthenium carbene catalysts, can solve the problems of low reaction temperature, long reaction time, low product yield, unsuitable for industrialized implementation, etc., and achieve high product yield and good product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
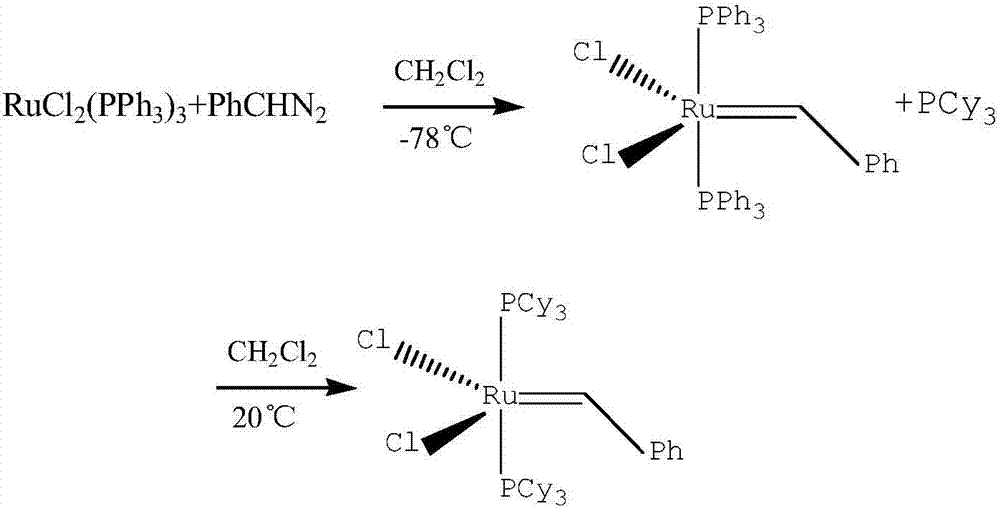
[0020] The invention provides a method for preparing a ruthenium carbene catalyst, the method comprising: (1) under the protection of an inert gas and in the presence of an organic solvent, at a temperature of -20°C to 0°C, diazobenzyl and dihalogenated tetrakis( Triphenylphosphine) ruthenium is subjected to the first reaction for 15-60min; (2) under the protection of an inert gas, the reaction product obtained in step (1) is subjected to the second reaction with tricyclohexylphosphine to obtain two-tricyclohexylphosphine- Phenylidene-dichlororuthenium complex.
[0021] In the present invention, the chemical formula of dihalogenated tetrakis (triphenylphosphine) ruthenium is RuX 2 (PPh 3 ) 4 (Ph represents phenyl), X=Cl, Br or I, preferably Cl. The chemical formula of diazobenzyl is PhCHN 2 , the structural formula is
[0022] According to a preferred embodiment of the present invention, in step (1), the molar ratio of diazobenzyl to dihalogenated tetrakis(triphenylphos...
Embodiment approach
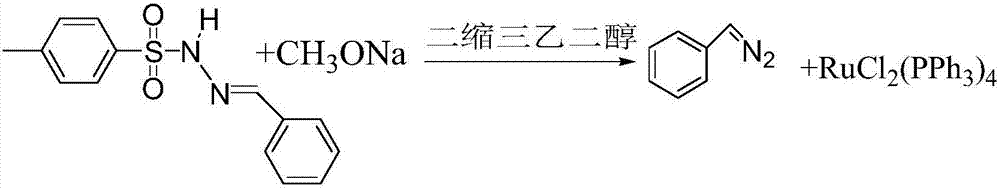
[0049] According to a preferred embodiment of the present invention, the mol ratio of benzaldehyde-p-toluenesulfonylhydrazone to the base is 1:(2~8); triethylene glycol and benzaldehyde-p-toluenesulfonate The molar ratio of acylhydrazone is (50-100):1, preferably the molar ratio of triethylene glycol to benzaldehyde-p-toluenesulfonylhydrazone is (63-94):1.
[0050] According to the present invention, preferably, the base is at least one of sodium methoxide, sodium ethoxide and sodium tert-butoxide.
[0051] A kind of preferred embodiment of the present invention, as mentioned above, the concrete process that obtains the organic solution that contains diazonium benzyl can be: benzaldehyde-p-toluenesulfonyl hydrazone, alkali and triethylene glycol are mixed, then Carry out the second synthesis at a reaction temperature of 40° C. to 80° C. for 1 to 2 hours, and remove the alcohol generated by the second synthesis by vacuuming in the middle. After the second synthesis, all the ma...
Embodiment 1
[0063] This example illustrates the preparation method of the ruthenium carbene catalyst of the present invention.
[0064] (1) Synthesis of dichloro tetrakis (triphenylphosphine) ruthenium
[0065] 0.6g (2.2mmol) of trihydrate ruthenium trichloride (RuCl 3 ·3H 2 (0, commercially available from Bailingwei) was dissolved in 150ml methanol and filtered to obtain a methanol solution of ruthenium trichloride trihydrate and the solution was refluxed for 5min under nitrogen protection, then the solution was cooled to 0°C; 3.6g (13.7mmol) Triphenylphosphine (P(Ph) 3 , commercially available from Bailingwei) was added to the above-mentioned cooled solution for the first synthesis, and a dark brown product solution was obtained. The product solution was stirred at about 25° C. under a nitrogen atmosphere for 24 hours, and then dark brown was collected under nitrogen protection. Crystallization product, this crystallization product is washed with the methanol of degassing, ether, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com