Solid broadband green-light emission crystal material and preparation method thereof
A crystal material and broadband technology, applied in the field of solid-state broadband green light-emitting crystal materials and their preparation, can solve problems affecting device performance and achieve enhanced luminous performance, easy control, and less weathering effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of solid-state broadband green light emitting crystal material (Compound I)
[0029]
[0030] Dissolve 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)acrolein (30mmol) and 4-aminoantipyrine (30mmol) in 100ml In the water ethanol, 0.5 ml of glacial acetic acid was added dropwise with rapid stirring, and the reaction was refluxed for 3 hours under stirring; then, the ethanol was removed by rotary evaporation, and the obtained solid matter was filtered under reduced pressure and dried to obtain the target product; the crude product was reconstituted with methanol or ethanol After two crystallizations, ethanol was used as a solvent, and the crystals were naturally volatilized at room temperature. After 10 days, light brown massive crystals suitable for X-ray single crystal diffraction measurement were obtained, and the yield was 87%.
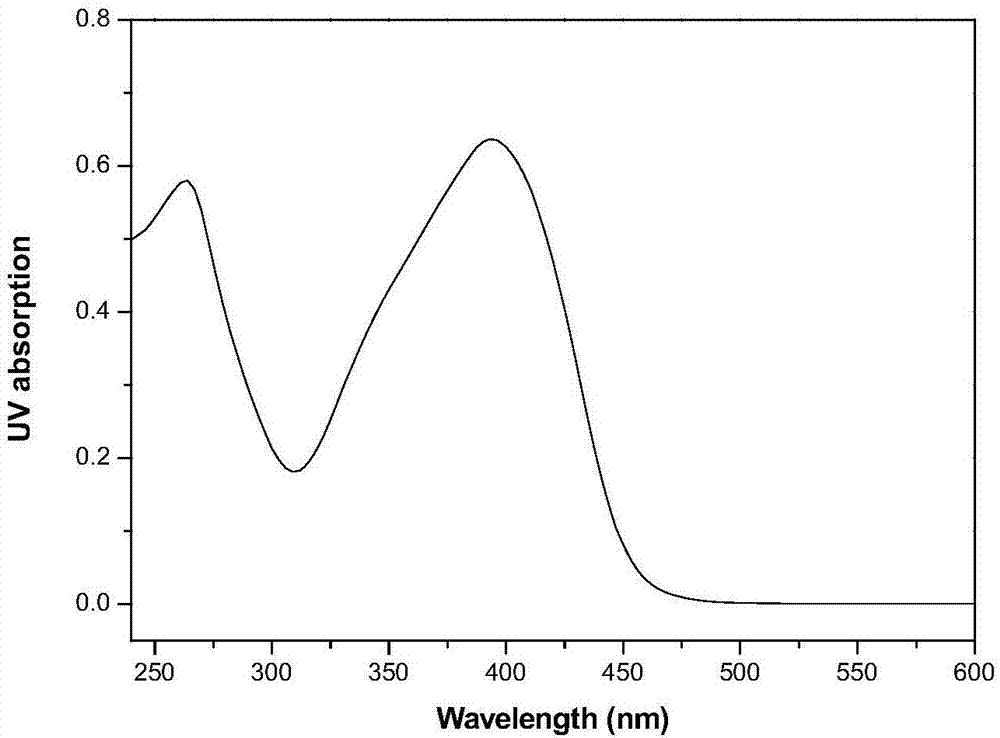
[0031] The obtained product was determined to be the target product by proton nuclear magnetic resonance spectrum, carbon n...
Embodiment 2
[0033] Example 2: Synthesis of solid-state broadband green light emitting crystal material (Compound I)
[0034] Obtained according to the method of Example 1, except that 3-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)acrolein and 4-aminoantipyrine The molar ratio of is 1:1.1, and the organic solvent used in the reaction is chloroform.
Embodiment 3
[0035] Example 3: Crystal structure determination
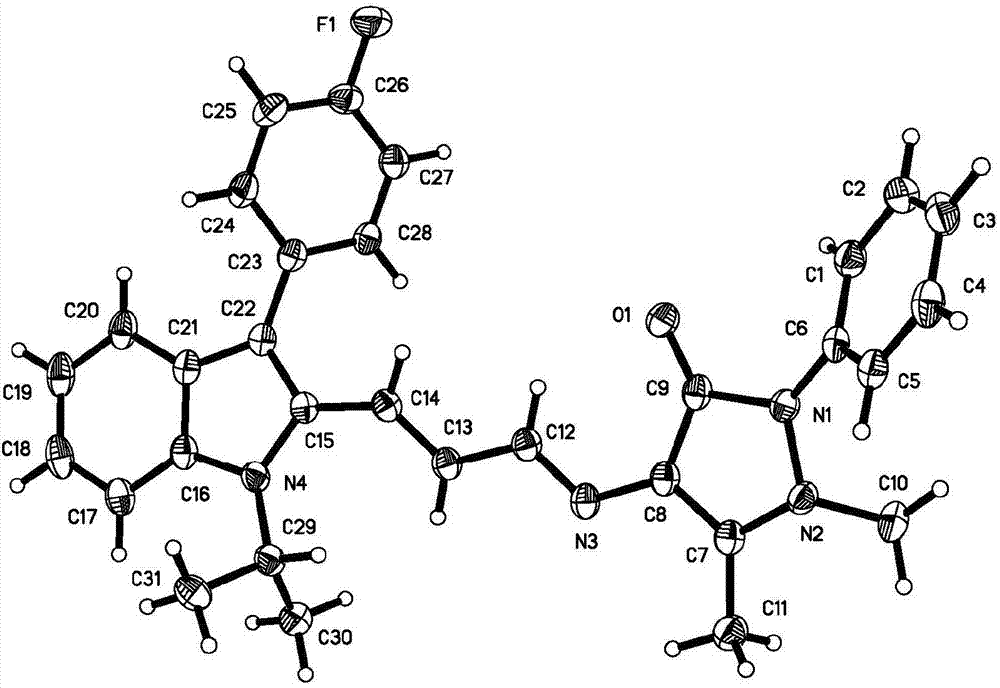
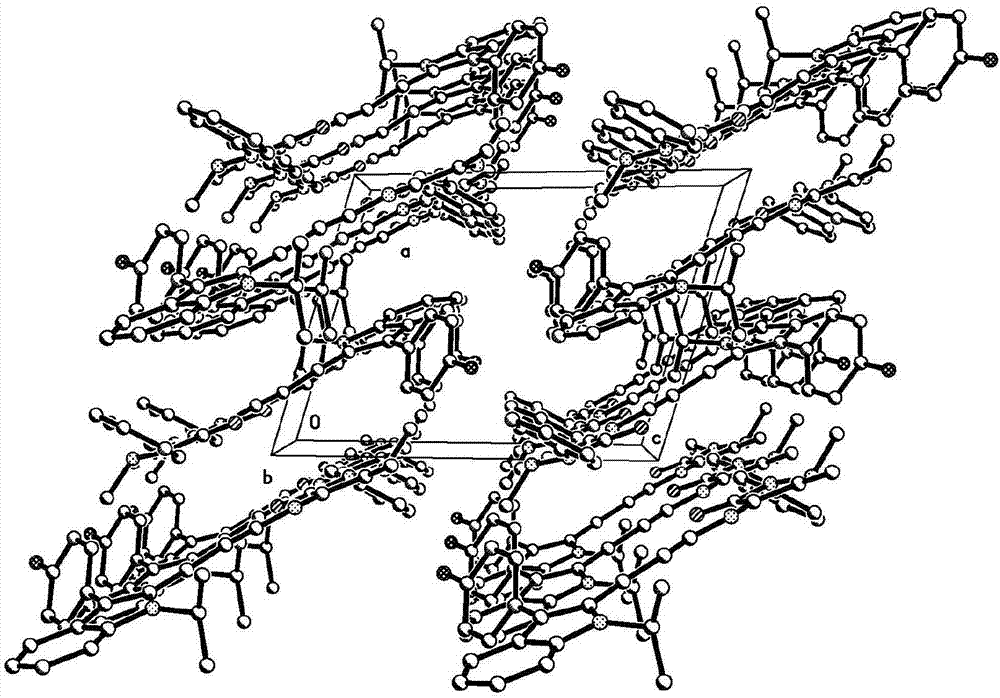
[0036] In order to further determine the molecular structure of the crystal material, the crystal of the solid broadband green light emitting crystal material prepared in Example 1 was analyzed by X-ray single crystal structure. Select a single crystal of a suitable size under a microscope. The size of the single crystal used for single crystal structure determination is 0.400x 0.340x 0.270mm, which is light brown. The crystal structure determination was carried out on the Bruker Smart1000CCD X-ray single crystal diffractometer, using Cu K monochromated by graphite α Rays Diffraction data was collected at 150(2)K. The crystal structure was solved by the direct method, and the software used was SHELXS-2013 and SHELXL-2014. All non-hydrogen atoms are obtained by difference Fourier synthesis and difference electron density function correction, and all hydrogen atom coordinates are obtained from the difference electron density fun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com