Polymer-drug conjugate based on a polyisoolefin-based copolymer
A conjugate and polymer technology, applied in the field of polymer-drug conjugates, can solve the problems of uropathogen species attachment, coating delamination, and stent life reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0047] Materials and methods:
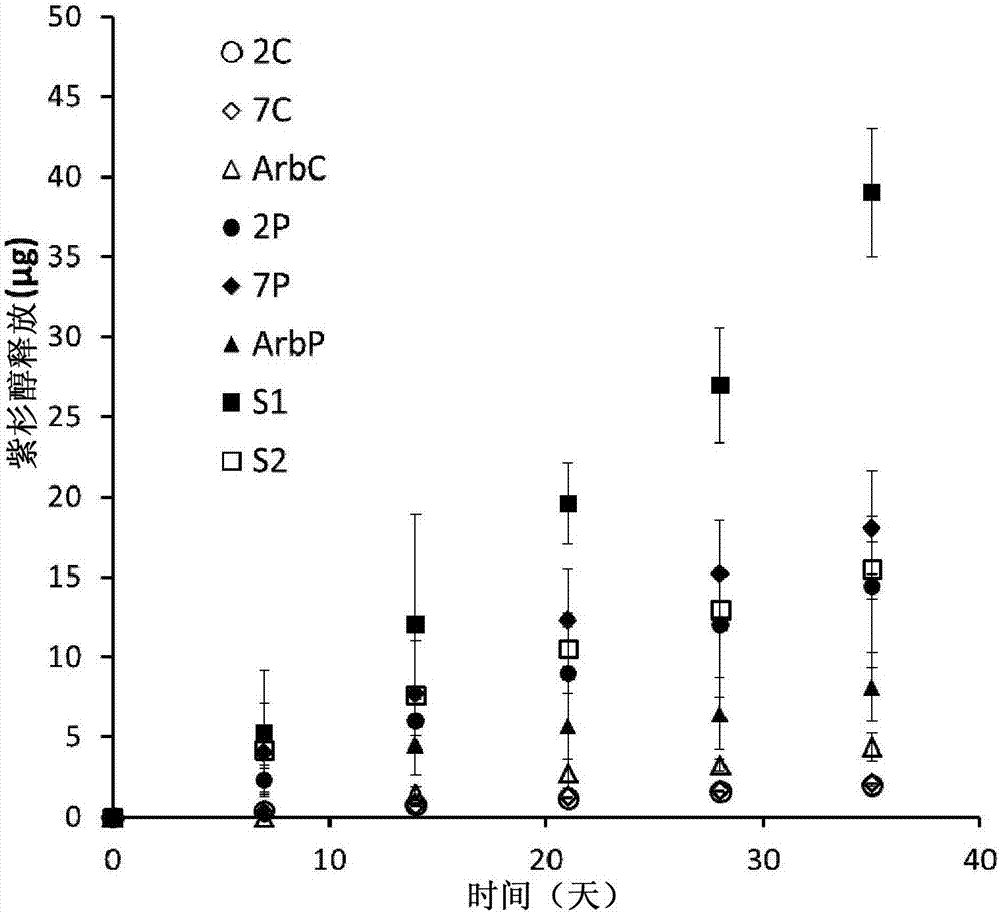
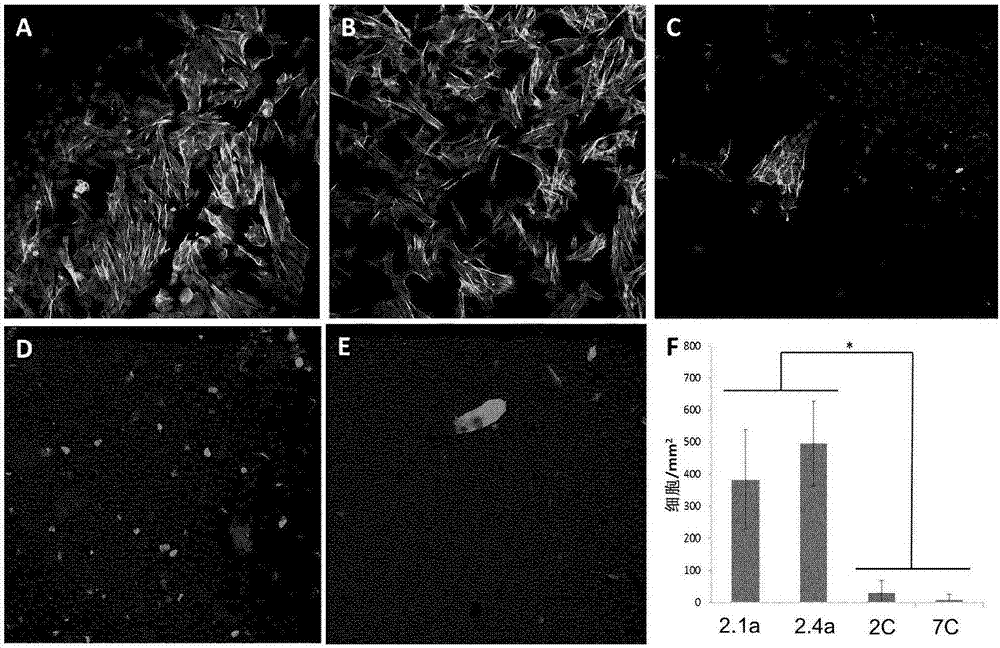
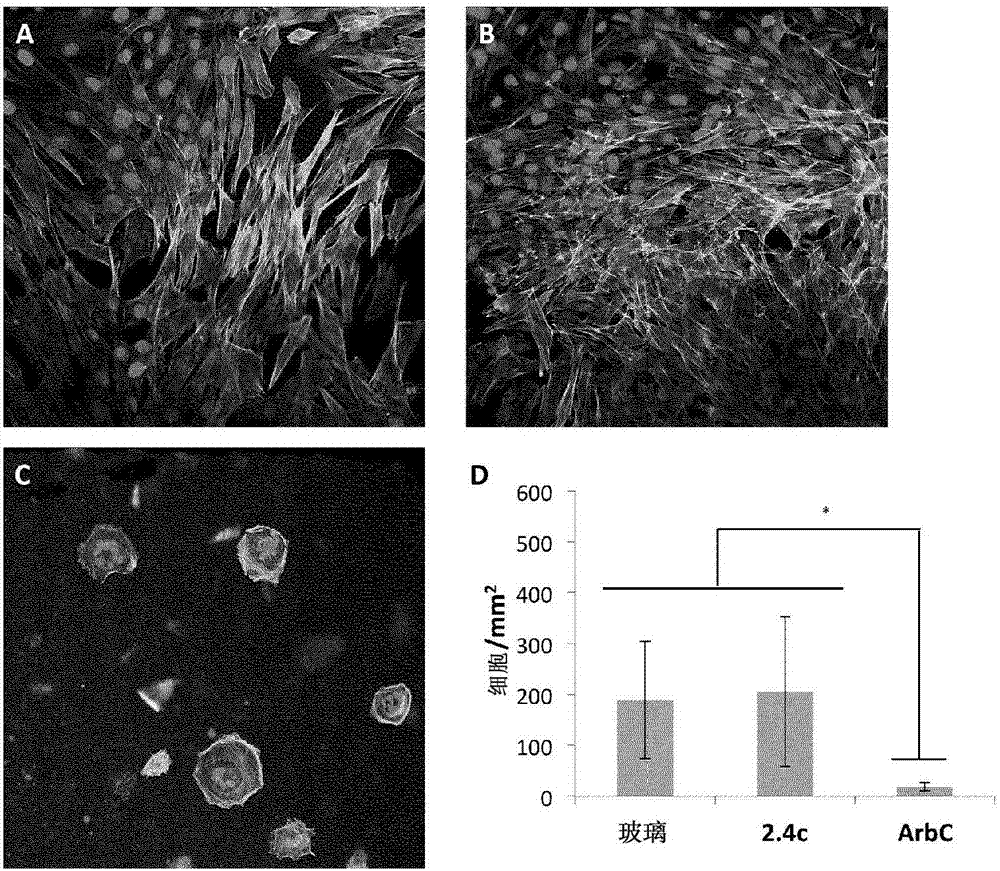
[0048] LANXESS Butyl 402(M w =4.69x10 5 g / mol, PDI=2.4, polymer 2.1a) and butyl rubber containing 7 mol% isoprene (M w =1.05x10 6 g / mole, PDI=3.3, Polymer 2.1b) was obtained from LANXESS Inc. With 6mol% isoprene (M w =216 kDa, PDI=1.4) dendritic poly(isobutylene-co-isoprene) was prepared as previously reported (Puskas 2009) and provided by LANXESS Inc. Paclitaxel was purchased from LC laboratories (Woburn, Ma). Solvents were purchased from Caledon and all other chemicals were purchased from Sigma-Aldrich and used without further purification unless otherwise stated. Dry toluene was obtained from a solvent purification system. in CDCl 3 obtained at 400MHz in 1 H NMR spectrum. NMR chemical shifts (δ) are reported in ppm and relative to CDCl 3 The residual solvent signal (δ7.26) was calibrated. Using a Bruker Tensor 27 instrument, as a NaCl plate from CH 2 Cl 2 Infrared spectra of the films were obtained. Using a Waters 515 pump in T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com