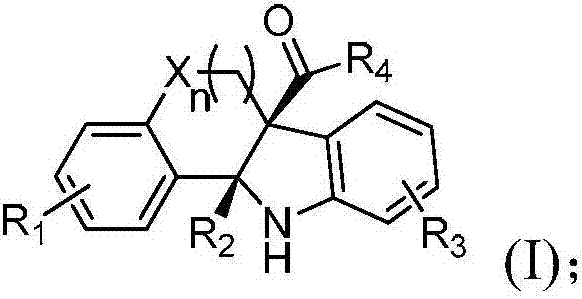
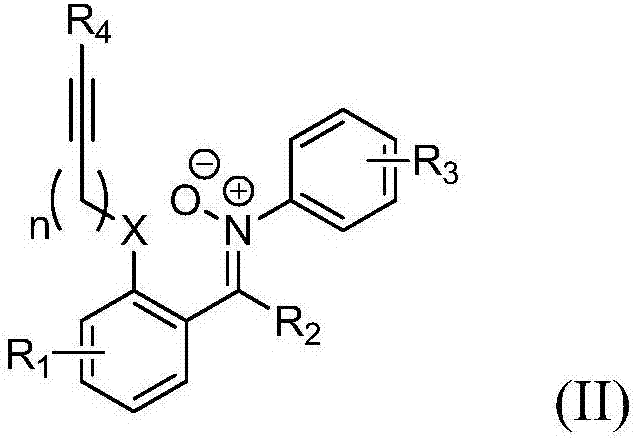
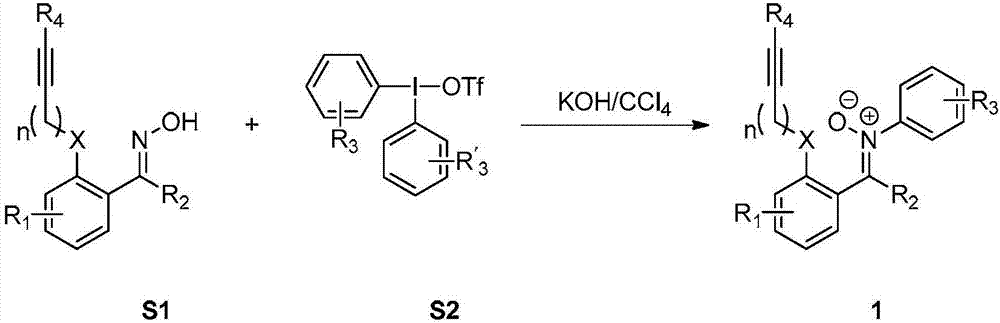
2,3-condensed ring indoline derivative and synthesizing method and application thereof
A compound and unsubstituted technology, applied in the field of medicine, can solve the problems of poor compatibility of functional groups, limited substrates, complex raw materials, etc., and achieve the effects of high yield, simple and easy-to-control synthetic method, and short cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesize the 2,3-fused ring indoline derivatives of the present invention according to the following synthetic route.
[0049]
[0050] 2a:R 1 =H,R 2 =PhCH=CH,R 3 =H,R 4 =H,n=1;
[0051] 2b:R 1 =H,R 2 =PhCH=CH,R 3 =4-OMe,R 4 =H,n=1;
[0052] 2c:R 1 =H,R 2 =PhCH=CH,R 3 =4-Me,R 4 =H,n=1;
[0053] 2d:R 1 =H,R 2 =PhCH=CH,R 3 =4-tBu,R 4 =H,n=1;
[0054] 2e:R 1 =H,R 2 =PhCH=CH,R 3 =4-Cl,R 4 =H,n=1;
[0055] 2f:R 1 =H,R 2 =PhCH=CH,R 3 =4-F,R 4 =H,n=1;
[0056] 2g:R 1 =H,R 2 =PhCH=CH,R 3 = 4-CF 3 , R 4 =H,n=1;
[0057] 2h:R 1 =H,R 2 =PhCH=CH,R 3 = 4-CO 2 Me,R 4 =H,n=1;
[0058] 2i:R 1 =H,R 2 =PhCH=CH,R 3 =3-OMe,R 4 =H,n=1;
[0059] 2j:R 1 =H,R 2 =PhCH=CH,R 3 = 3-NO 2 , R 4 =H,n=1;
[0060] 2k:R 1 =H,R 2 =PhCH=CH,R 3 =3,5-Me,R 4 =H,n=1;
[0061] 2l:R 1 =H,R 2 =PhCH=CH,R 3 =2-Me,R 4 =H,n=1;
[0062] 2ab:R 1 =H,R 2 =(4-OMe)C 6 h 4 CH=CH,R 3 =H,R 4 =H,n=1;
[0063] 2ac:R 1 =H,R 2 =(4-Me)C 6 h 4 CH=CH,R 3 =...
Embodiment 2
[0142] Synthesize the 2,3-fused ring indoline derivatives of the present invention according to the following synthetic route.
[0143]
[0144] 2ba:R 1 =4-Br,R 2 =PhCH=CH,R 3 =4-Br,R 4 =H,n=1;
[0145] 2bb:R 1 =4-Br,R 2 =PhCH=CH,R 3 =3-Br,R 4 =H,n=1;
[0146] 2bc:R 1 =4-Br,R 2 =(3-Br)C 6 h 4 CH=CH,R 3 =4-Cl,R 4 =H,n=1;
[0147] 2bd:R 1 =4-Br,R 2 =(4-Me)C 6 h 4 CH=CH,R 3 =4-Cl,R 4 =Me,n=1;
[0148] 2be:R 1 =3-Cl,R 2 =Me,R 3 =4-Me,R 4 =H,n=1;
[0149] 2bf:R 1 =4-Br,R 2 =Me,R 3 =4-Me,R 4 =4-ClC 6 h 4 ,n=1;
[0150] 2bg:R 1 =3-Cl,R 2 =Me,R 3 =4-Me,R 4 =Me,n=1;
[0151] 2bh:R 1 =4-Br,R 2 =H,R 3 =4-Br,R 4 =H,n=1;
[0152] 2bi:R 1 =4-Br,R 2 =H,R 3 =3-Cl,R 4 =Me,n=2;
[0153] 2bj:R 1 =4-Br,R 2 =Me,R 3 =4-Br,R 4 =Me, n=1, X=N.
[0154] Take N-aryl α, β-unsaturated nitrone substrate 1 (0.2mmol) and place it in a reaction tube, add 2mL of organic solvent (wherein the organic solvents used for target 2ba-2bd are acetone, chloroform, n...
experiment example 1
[0175] Experimental Example 1: The 2,3-fused ring indoline derivatives of the present invention were tested for their inhibitory activity against various human tumor strains in vitro:
[0176] (1) Cell culture: MGC-803, HepG-2, NCI-H460, SKOV3, T24, 7702 cells were cultured in a medium containing 10% (volume ratio) fetal bovine serum and 1% (volume ratio) double antibody (containing penicillin and Streptomycin) in DMEM medium at 37°C, 5% CO 2 and 95% air incubator, and change the medium every other day. After the cells were confluent, they were subcultured and frozen.
[0177] (2) Seed plate: Take cells in the logarithmic growth phase, remove the old medium, wash twice with PBS, digest the cells with trypsin, add new medium after the cells become round, stop the digestion of the cells and pipette the suspended cells to prepare into a single cell suspension. Take an appropriate amount of cell suspension, add a certain amount of medium to dilute, inoculate into a 96-well plat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com