Drug nano preparation and preparation method thereof
A nano-preparation and drug technology, which is applied in the field of preparation of nano-drug-loaded preparations, can solve the problems of high expression level of transcription factor HIF1alpha in tumor cells, impermeability to tumor tissue, etc., to achieve increased penetration, increased toxicity, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
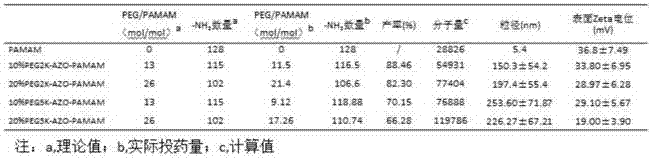
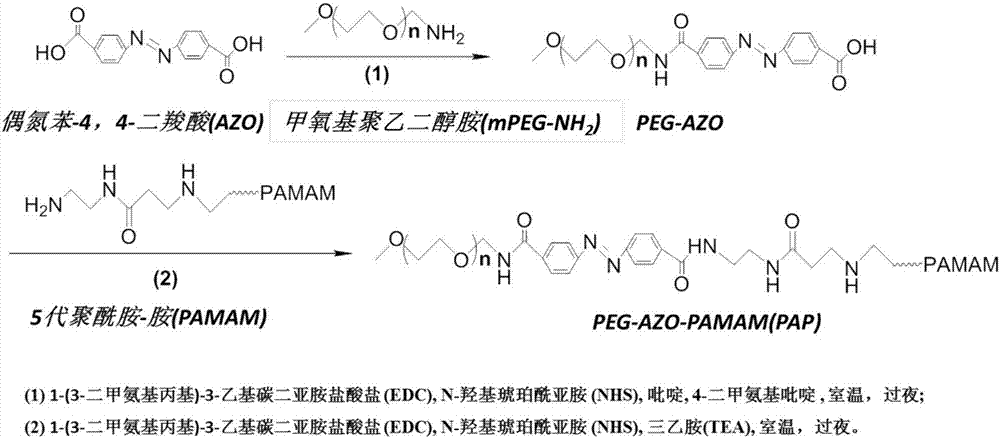
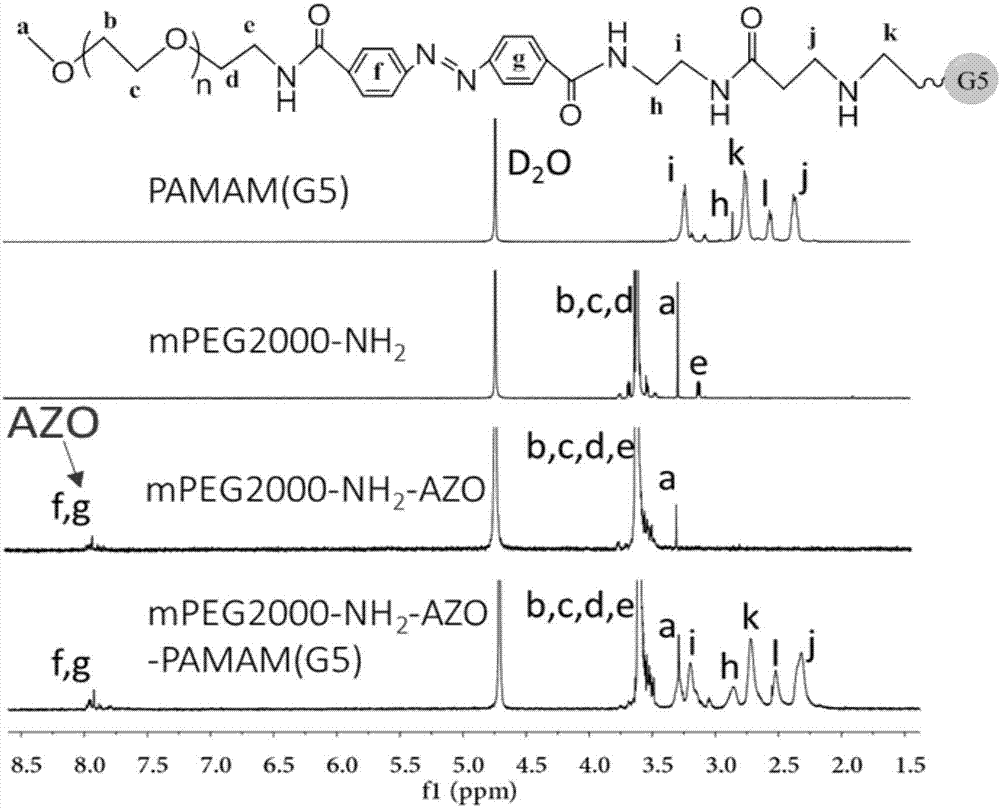
[0052] 1. Preparation of 20% PEG2K-AZO-PAMAM (20% PEG grafting rate, PEG molecular weight 2000) nanocarrier
[0053] 1) Take 5mg of AZO, ultrasonically dissolve it in 3ml of pyridine, add 4.37mg of EDC·HCl, 2.56mg of NHS and 0.23mg of 4-DMAP, adjust the pH of the solution to 5 with 1mol / L HCl, and stir at room temperature for 2 hours to activate the carboxyl group of AZO .
[0054] 2) Dissolve 37mg of mPEG-NH2 (MW=2000) in 1ml of pyridine; slowly add the mPEG pyridine solution into the AZO solution under magnetic stirring, and add 5 μl of triethylamine dropwise; stir overnight at room temperature.
[0055] 3) Remove the pyridine solution by vacuum rotary evaporation at 50°C to obtain an orange-yellow film product. Add 10ml of pure water to redissolve, centrifuge at 3000rpm for 5min to remove free AZO precipitate, and obtain mPEG-AZO aqueous solution. Add 4.37mg EDC·HCl, 2.56mg NHS, adjust the pH of the solution to 5 with 1mol / L HCl, stir at room temperature for 2 hours, and ...
Embodiment 2
[0065] This embodiment removes mPEG-NH 2 The molecular weight of PEG5K-AZO-PAMAM is 5000, and the dosage is 92.5 mg, and other steps are the same as in Example 1 to prepare 20% PEG5K-AZO-PAMAM (20% PEG grafting ratio, PEG molecular weight 5000).
Embodiment 3
[0067] In this example, except that the dosage of 20.5% PAMAM (G5.0) was 200 μl, other steps were the same as in Example 1 to prepare 10% PEG2K-AZO-PAMAM (10% PEG grafting ratio, PEG molecular weight 2000).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com