Antibacterial finishing method of cotton fabrics
An antibacterial finishing and cotton fabric technology, applied in fiber treatment, plant fiber, biochemical fiber treatment, etc., can solve problems such as weak binding force, not washable, affecting human health, etc., and achieve the effect of uniform dispersion and good antibacterial performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
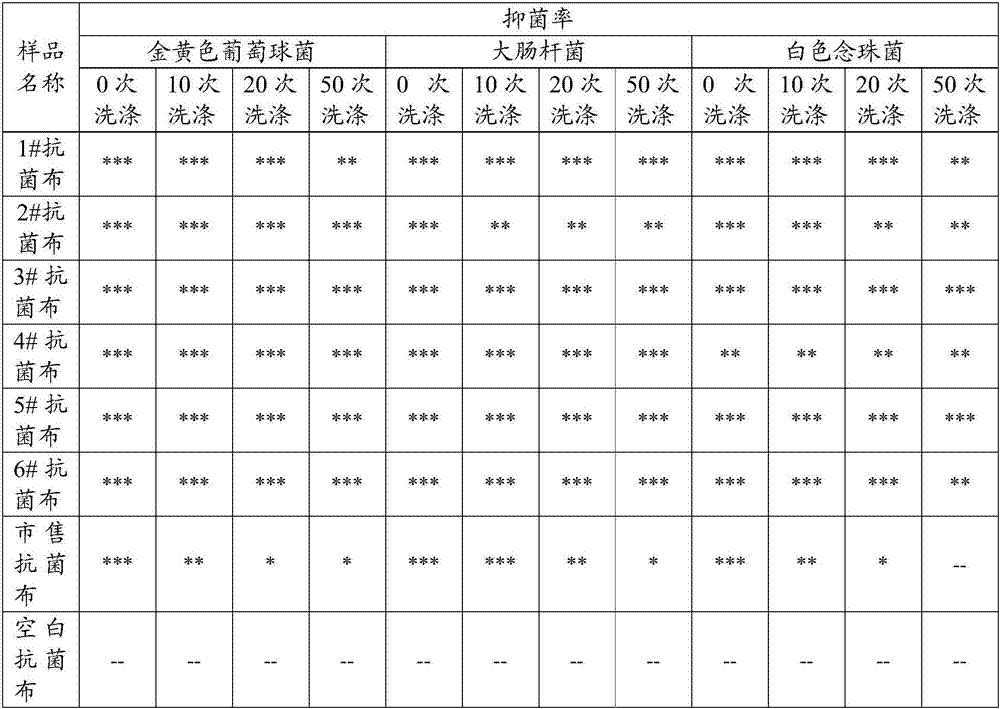
Examples
Embodiment 1
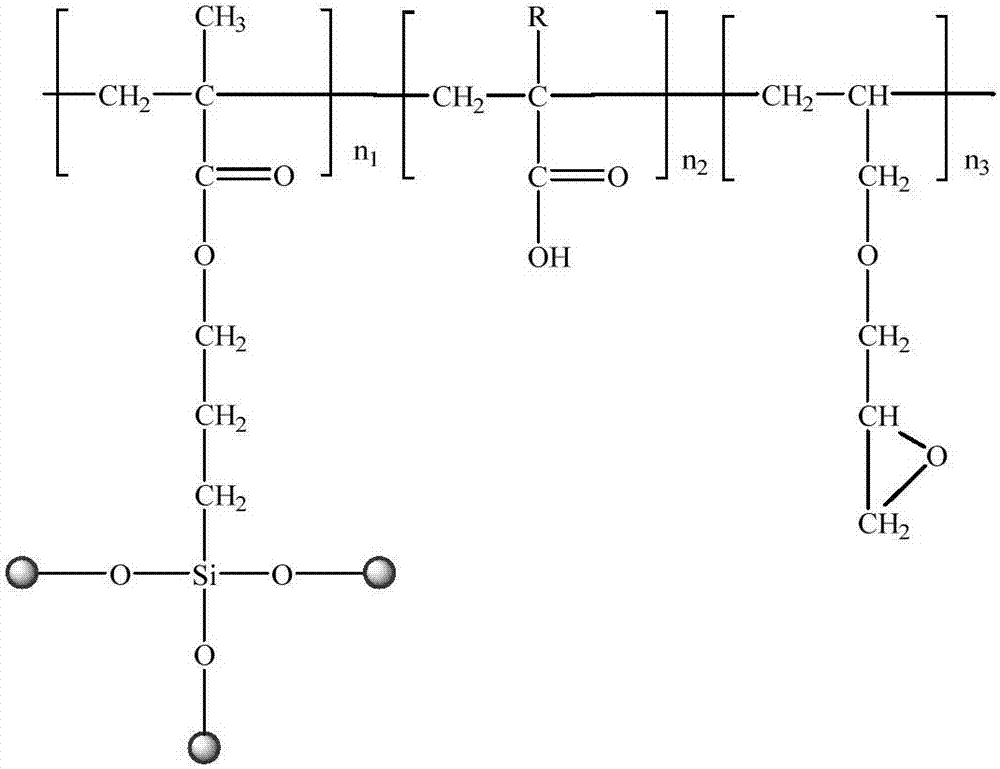
[0107] (1) Preparation of nano-zinc oxide composite solution: Mix 1.0g nano-zinc oxide, 40g ethanol, 18g water and 4g γ-methacryloxypropyltrimethoxysilane evenly, ultrasonically disperse for 1h, and heat at 60°C The water bath was refluxed for 12 hours, then cooled to room temperature, and the product was centrifuged and washed to obtain active nanometer zinc oxide. Dissolve 4g of ammonium persulfate in 10g of deionized water, and divide it into two equal parts, then add 4.0g of active nano-zinc oxide, 50g of deionized water, 12g of acrylic acid, 4.0g of allyl glycidyl ether and 7g of potassium persulfate solution to the In a three-neck flask, stir evenly, then heat in a water bath to 80°C, and react for 20 minutes; then add dropwise 12g of acrylic acid, 4.0g of allyl glycidyl ether, and 7g of potassium persulfate solution, keep stirring for 4.0h, then cool down to room temperature, that is Obtain nano-zinc oxide complex solution.
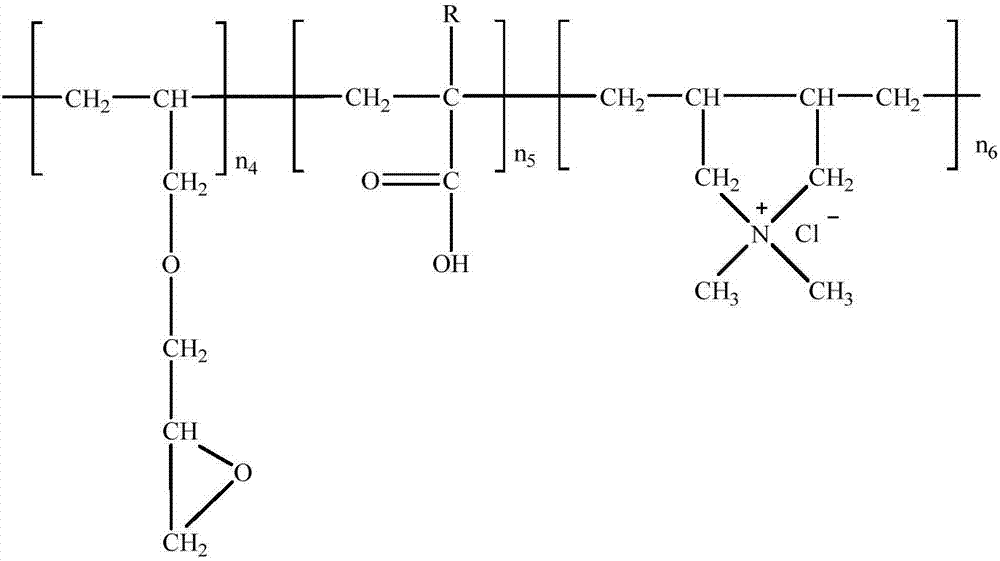
[0108] (2) Synthesis of quaternary ammonium...
Embodiment 2
[0110] (1) Preparation of nano-zinc oxide composite solution: Mix 1.0g nano-zinc oxide, 40g ethanol, 18g water and 4g γ-methacryloxypropyltrimethoxysilane evenly, ultrasonically disperse for 1h, and heat at 60°C The water bath was refluxed for 12 hours, then cooled to room temperature, and the product was centrifuged and washed to obtain active nanometer zinc oxide. Dissolve 4g of ammonium persulfate in 10g of deionized water, and divide it into two equal parts, then add 4.0g of active nano-zinc oxide, 50g of deionized water, 12g of acrylic acid, 4.0g of allyl glycidyl ether and 7g of potassium persulfate solution to the In a three-neck flask, stir evenly, then heat in a water bath to 80°C, and react for 20 minutes; then add dropwise 12g of acrylic acid, 4.0g of allyl glycidyl ether, and 7g of potassium persulfate solution, keep stirring for 4.0h, then cool down to room temperature, that is Obtain nano-zinc oxide complex solution.
[0111] (2) Synthesis of quaternary ammonium...
Embodiment 3
[0113] (1) Preparation of nano-zinc oxide composite solution: Mix 1.0g nano-zinc oxide, 40g ethanol, 18g water and 4g γ-methacryloxypropyltrimethoxysilane evenly, ultrasonically disperse for 1h, and heat at 60°C The water bath was refluxed for 12 hours, then cooled to room temperature, and the product was centrifuged and washed to obtain active nanometer zinc oxide. Dissolve 4g of ammonium persulfate in 10g of deionized water, and divide it into two equal parts, then add 4.0g of active nano-zinc oxide, 50g of deionized water, 12g of acrylic acid, 4.0g of allyl glycidyl ether and 7g of potassium persulfate solution to the In a three-neck flask, stir evenly, then heat in a water bath to 80°C, and react for 20 minutes; then add dropwise 12g of acrylic acid, 4.0g of allyl glycidyl ether, and 7g of potassium persulfate solution, keep stirring for 4.0h, then cool down to room temperature, that is Obtain nano-zinc oxide complex solution.
[0114] (2) Synthesis of quaternary ammonium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com