Method for measuring content of calycosin-7-glucoside in lung tonifying and blood circulation promoting capsules
A technology for glucosinolates and glucosinolates, which is applied to measuring devices, instruments, scientific instruments, etc., can solve the problems of undocumented methods for measuring the content of glucosinolates on glucosinolates, and achieves strong specificity, good accuracy, feasibility, and precision. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The selection of embodiment 1 chromatographic conditions
[0053] 1. Mobile phase
[0054] Referring to the 2015 edition of "Chinese Pharmacopoeia" in Astragalus medicinal materials [Content Determination] Calycosin glucoside, acetonitrile was used as mobile phase A, 0.2% formic acid solution was used as mobile phase B, and when the elution gradient was adjusted to the following table 2, Calycosin Both flavonoid glucoside and calycosin are well separated.
[0055] Table 2 Mobile phase elution gradient
[0056]
[0057] 2. Detection wavelength
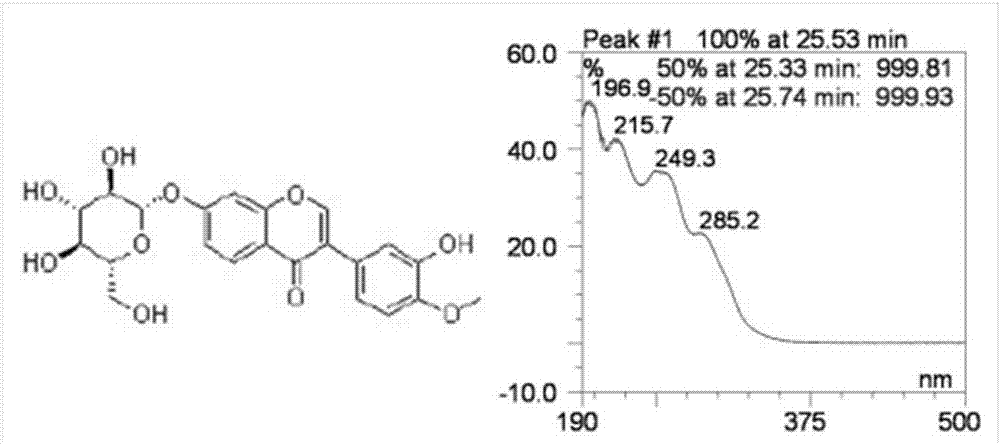
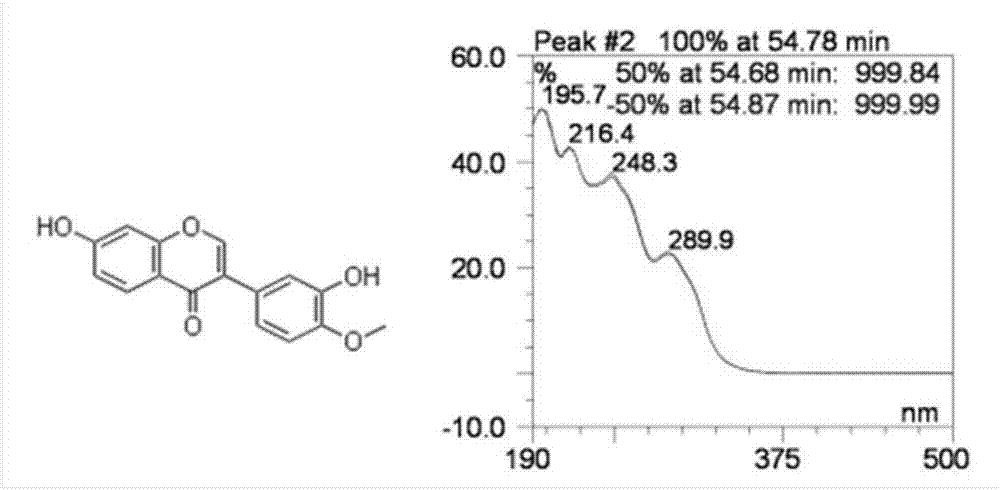
[0058] Take the methanol solution of calycocoside glucoside and calycocoside reference substance to measure the ultraviolet absorption spectrum, both of them have large absorption at 260nm, so 260nm is selected as the detection wavelength. See attached figure 1 And attached figure 2 .
Embodiment 2
[0059] The preparation of embodiment 2 sample
[0060] 1. Preparation of reference substance solution: Take appropriate amount of actcocoside glucoside and acteocoside reference substance respectively, weigh them accurately, add 50% methanol to make a mixed solution containing 30 μg of actcocoside glucoside and 20 μg of actcocoside per 1 ml, and get ready.
[0061] 2. Preparation of the test solution: Take the content of this product, grind it finely, take about 1.2g, accurately weigh it, put it in a stoppered conical flask, add 50ml of methanol precisely, weigh it, heat and reflux for 1 hour, put Cool, weigh again, make up for the lost weight with methanol, shake well, filter, accurately measure 25ml of the subsequent filtrate, recover the solvent to dryness, add 50% methanol to the residue to dissolve, transfer to a 5ml measuring bottle, add 50% Methanol to the mark, shake well, that is.
[0062] 3. The choice of solvent for the preparation of the test solution: This test...
Embodiment 3
[0068] Embodiment 3 system suitability test
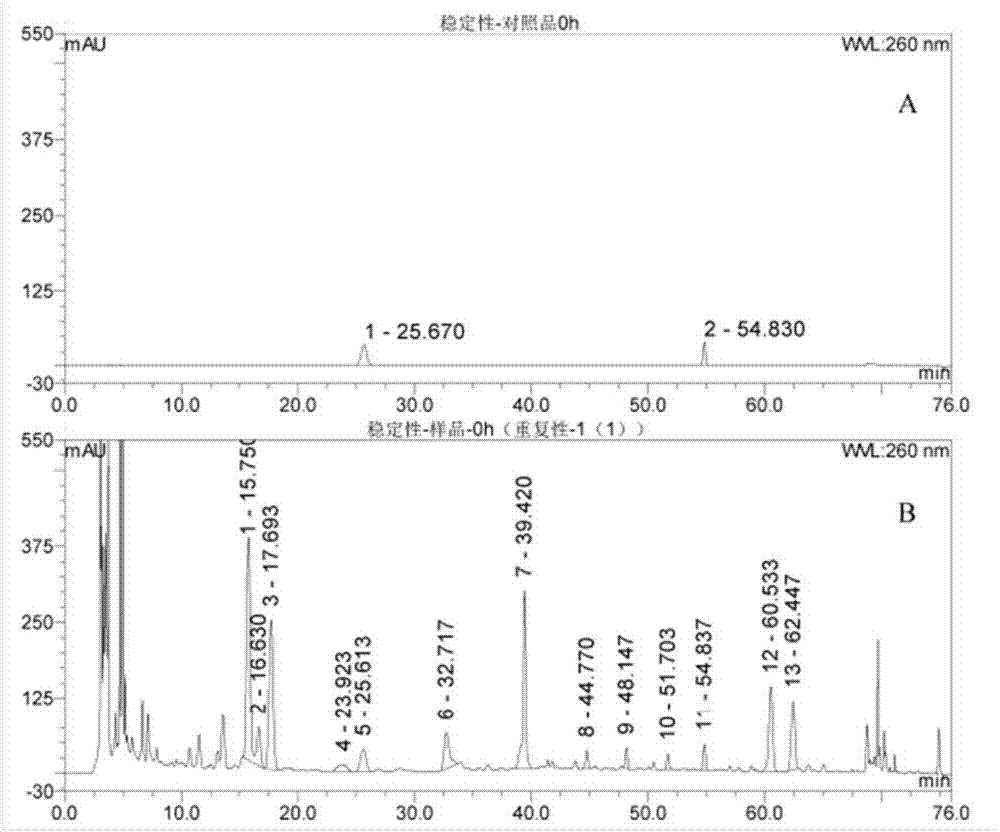
[0069] Draw the reference substance solution and the test solution into the liquid chromatograph for determination. Under this chromatographic condition, calycocoside glucoside, calycocoside and other components achieve baseline separation, and the resolution R>1.5. According to different chromatographic column theoretical plates (Hitachi column is about 13,000; Sepax column is about 15,000; Welch column is about 17,000; Thermo column is about 12,000), and the number of theoretical plates for this sample is not less than 12,000 based on calycosin glucoside. See Table 5, Table 6 and Appendix image 3 .
[0070] Table 5 System Adaptability Data (Calycosin Glucoside Reference Substance)
[0071]
[0072] Table 6 system suitability data (calycosin reference substance)
[0073]
[0074] Note: The resolution in the table is the resolution data in the precision
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com