Triple and four-prevention subunit vaccine for sheep and preparation method thereof
A subunit vaccine and sheep triplet technology, applied in vaccines, veterinary vaccines, multivalent vaccines, etc., can solve the problems of high immune side effects, high cost, difficult to control the quality of culture substrates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Cloning of Clostridium welchii C58-1 strain β-ε and Clostridium putrefaction C55-2 strain α-toxin and construction of expression vector
[0070] 1.1 β-ε toxin gene cloning and construction of expression vector
[0071] According to the β and ε sequences of C58-1 strain, 6 specific primers were designed. The primer sequences are:
[0072] pεb1947s1:GCTTTTCCTAGGGATG;
[0073] pεb1947s2:AATGATATAGGTAAAACTACTAC;
[0074] pεb1947r1:CTTCGCCGCCGCTTCCGCTTTTATTCCTGGTGCC;
[0075] pεb1947r2:
[0076] GGGGTCGACCTATATCATTCGCGCCGCCGCTTCTTTCGCCGC;
[0077] pεb1947r3:TACCTATATCATTCGCTTTCG;
[0078] pεb1947r4: TATTTTGAATGTAAATATATGAC. The underlined part is the AvrII and SalI restriction sites of the β and ε sequences, and the italic and bold parts are the connecting peptide sequences.
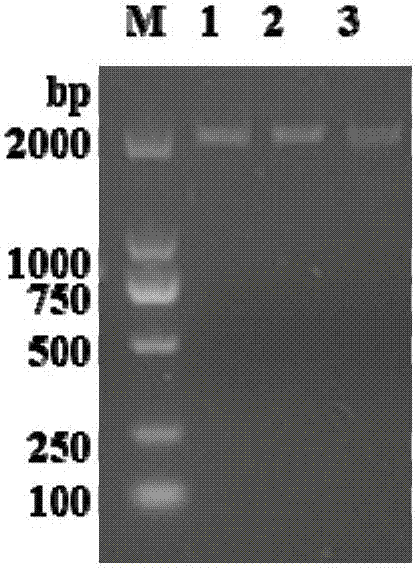
[0079] Utilize 1% agarose gel electrophoresis to detect PCR amplification product, the result is as follows figure 1 shown. The results showed that there was a specific band smalle...
Embodiment 2
[0086] Example 2 Expression and purification of β-ε and α toxin
[0087] 1.2 Expression and purification of β-ε toxin
[0088] Mix pET1947 and BL21(DE3)pLysS competent cells for transformation to obtain recombinant strain BL21(DE3)-pET1947; add recombinant strain BL21(DE3)-pET1947 to LB / Amp medium at a ratio of 1:100, Shake the bacteria on a shaker at 37°C (180r / min) for 3h. When OD600 reached 0.4, IPTG with a concentration of 1 mol / L was added, and an empty vector control group was set at the same time, and induced on a shaker at 37°C for 5 hours at a rotational speed of 180r / min. Afterwards, the induced bacterial solution was centrifuged at 4000r / min for 20min, resuspended in PBS, and ultrasonically broken to a clear state. Centrifuge at 12000r / min for 10min at 4°C. Each 1 mL of the bacterial liquid induced by ultrasonic disruption was centrifuged at 10,000 r / min for 10 min, and the precipitate and supernatant were collected for SDS-PAGE electrophoresis analysis to analyz...
Embodiment 3
[0095] Example 3 Research on the immune protection of β-ε and α toxin proteins on animals (according to the inspection standard of Sanbu sheep "triple and four-proof vaccine" of the Veterinary Pharmacopoeia of the People's Republic of China 2010 Edition)
[0096] 3.1 Mouse lethal activity test and results
[0097] Select gelatin buffer to dilute Clostridium putrefaction α toxin recombinant protein and Clostridium perfringens β-ε fusion protein, select three titers of 50, 100, and 150ug, and inject 2 mice intraperitoneally for each titer, Observe for 3 to 5 days. The dilution method and results are shown in Table 1 below. The test results showed that the α-toxin recombinant protein of Clostridium putrefaciens and the β-ε fusion protein of Clostridium perfringens were toxic to mice, and the MLD of mice injected intraperitoneally should be between 50-100ug.
[0098] Table 1 Toxicity detection of β-ε and α toxin proteins
[0099]
[0100]
[0101] Note: *Number of deaths / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com