R-4-hydroxyl-2-(2-hydroxy phenyl)-butyric acid as well as preparation and application thereof
A hydroxyphenyl, hydroxyl technology, applied in the field of organic compounds and pesticide applications, can solve the problem of no pesticide composition report, and achieve the effect of significant effect and inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of R-4-hydroxyl-2-(2-hydroxyphenyl)-butyric acid (hereinafter referred to as: compound of the present invention) shown in structural formula (I) of the present invention:
[0028] Take 5 kg of the above-ground parts of potato plants infected with Phytophthora infestans, dry them and crush them through a 40-mesh sieve as a raw material, use 90% (v / v) ethanol to reflux extract twice, each time for 3 hours, combine the ethanol extracts, and recover under reduced pressure Ethanol extract 200 grams. The extract is dissolved in 1000 milliliters of water to obtain an aqueous extract solution, and the aqueous extract solution is extracted with ethyl acetate equal to the volume of the aqueous extract solution to obtain 1000 ml of ethyl acetate extract, which is extracted 3 times in total and combined and extracted 3 times. After the ethyl acetate extract, it was concentrated under reduced pressure to obtain the ethyl acetate extract fraction. The ethyl acetate e...
Embodiment 2
[0031] Structural identification of the compounds of the present invention:
[0032] Optical rotation was measured by Jascommodel 1020 polarimeter (Horiba, Tokyo, Japan); nuclear magnetic resonance (1D and 2DNMR) was measured by AVANCE III-600 superconducting nuclear magnetic resonance (Bruker, Bremerhaven, Germany), using deuterated chloroform as solvent; High-resolution mass spectrometry (HRMS) was measured by LCMS-IT-TOF mass spectrometer (Shimadzu, Kyoto, Japan); thin-layer chromatography silica gel and column chromatography silica gel (200-300 mesh) were purchased from Qingdao Meigao and Qingdao Ocean Chemical Group Co., Ltd. company. Sephadex LH-20 gel was purchased from Pharmacia Fine Chemical Co., Ltd. (Uppsala, Sweden).
[0033] The relevant data and structural formula (1) of compound identification of the present invention are as follows:
[0034]
[0035] The above structural formula (I) is the structural formula of the compound of the present invention, and its ...
Embodiment 3
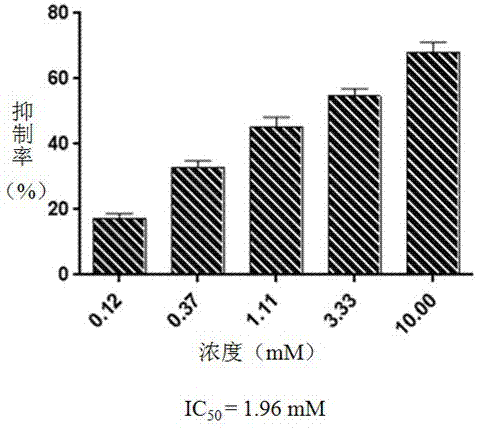
[0043] Anti-Phytophthora infestans activity test of the compound of the present invention
[0044] 1 Test method:
[0045] Inhibition of hyphae growth test: the compound of the present invention is about to be tested monomer compound with the rye culture medium that melts and is diluted to 1%, 0.5%, 0.25%, 0.125% and 0.0625% mass fraction different concentration, and fully mixes, with The rye culture medium without test samples was used as blank control. After the culture medium was cooled and solidified, a plate with a diameter of 0.6 cm of Phytophthora infestans was inoculated in the center of the plate, and the diameter of the colony was measured after culturing at 17°C for 5 days. Calculate the inhibitory activity of different concentrations of test samples on mycelial growth according to the following formula. Potato infestans (Phytophthora infestans) was purchased from Beijing Biobowell Biotechnology Co., Ltd., product number bio-78001.
[0046] Mycelial growth inhibi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com